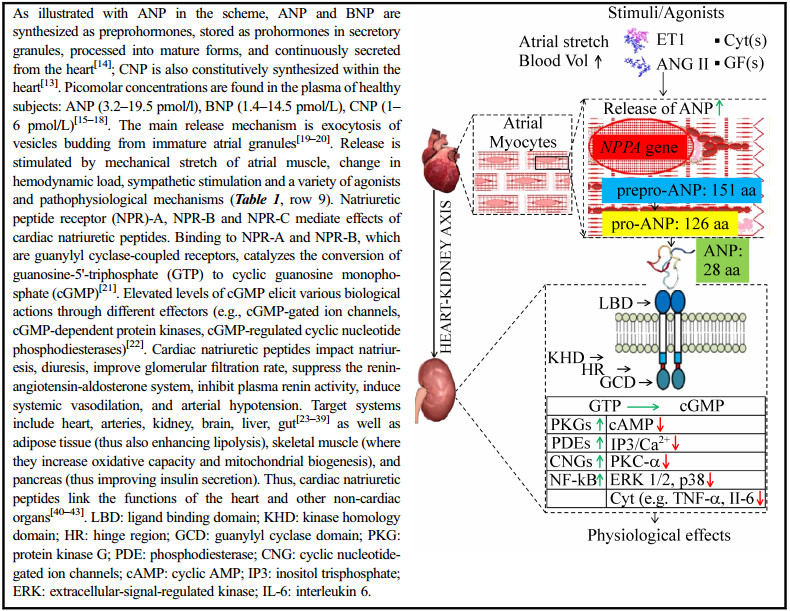
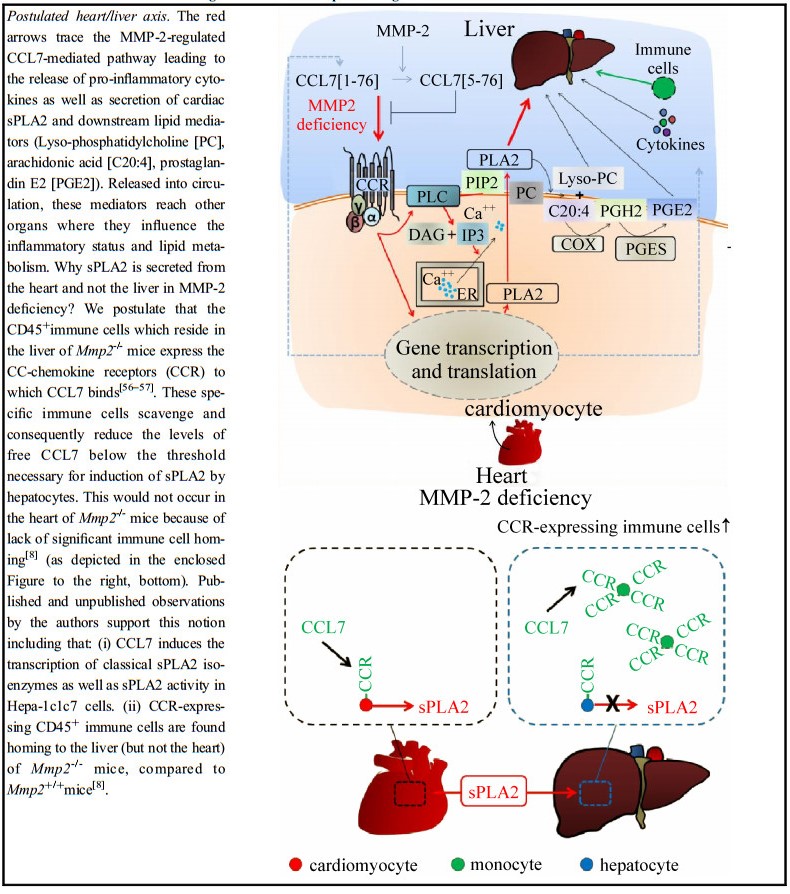
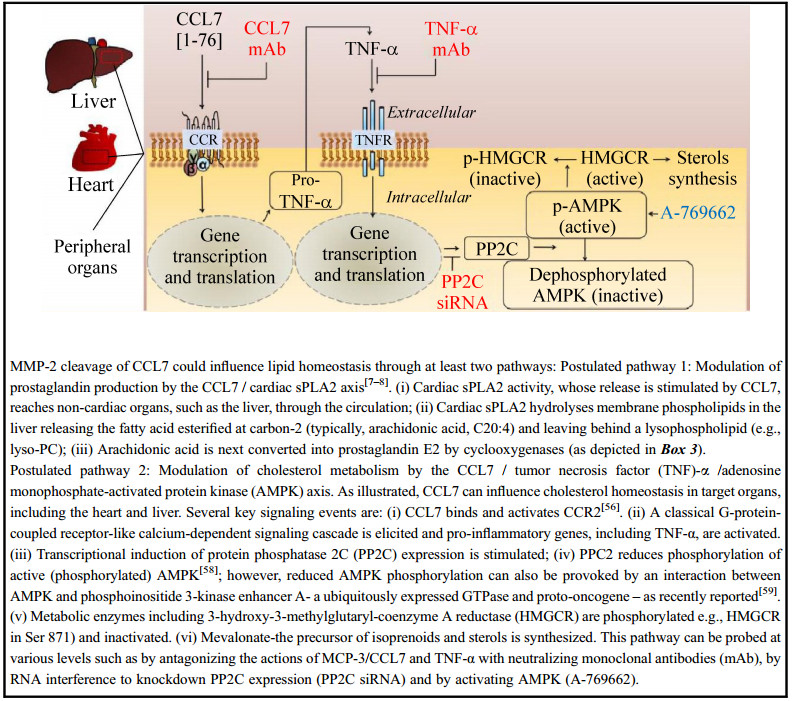
| [1] |
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013[J]. Lancet, 2015, 385(9963): 117-171. doi: 10.1016/S0140-6736(14)61682-2
|
| [2] |
de Bold AJ, Borenstein HB, Veress AT, et al. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats[J]. Life Sci, 1981, 28(1): 89-94. doi: 10.1016/0024-3205(81)90370-2
|
| [3] |
de Bold AJ, Salerno TA. Natriuretic activity of extracts obtained from hearts of different species and from various rat tissues[J]. Can J Physiol Pharmacol, 1983, 61(2): 127-130. doi: 10.1139/y83-018
|
| [4] |
de Bold AJ, Flynn TG. Cardionatrin I- a novel heart peptide with potent diuretic and natriuretic properties[J]. Life Sci, 1983, 33(3): 297-302. doi: 10.1016/0024-3205(83)90390-9
|
| [5] |
Flynn TG, Davies PL, Kennedy BP, et al. Alignment of rat cardionatrin sequences with the preprocardionatrin sequence from complementary DNA[J]. Science, 1985, 228(4697): 323- 325. doi: 10.1126/science.3157217
|
| [6] |
Grueter CE, van Rooij E, Johnson BA, et al. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13[J]. Cell, 2012, 149(3): 671-683. doi: 10.1016/j.cell.2012.03.029
|
| [7] |
|
| [8] |
|
| [9] |
Wang X, Berry E, Hernandez-Anzaldo S, et al. Matrix metalloproteinase-2 mediates a mechanism of metabolic cardioprotection consisting of negative regulation of the sterol regulatory element-binding protein-2/3-hydroxy-3-methylglutaryl-CoA reductase pathway in the heart[J]. Hypertension, 2015, 65(4):882-888. doi: 10.1161/HYPERTENSIONAHA.114.04989
|
| [10] |
Sudoh T, Kangawa K, Minamino N, et al. A new natriuretic peptide in porcine brain[J]. Nature, 1988, 332(6159): 78-81. doi: 10.1038/332078a0
|
| [11] |
Nakamura S, Naruse M, Naruse K, et al. Atrial natriuretic peptide and brain natriuretic peptide coexist in the secretory granules of human cardiac myocytes[J]. Am J Hypertens, 1991, 4(11): 909-912. doi: 10.1093/ajh/4.11.909
|
| [12] |
Clerico A, Giannoni A, Vittorini S, et al. Thirty years of the heart as an endocrine organ: physiological role and clinical utility of cardiac natriuretic hormones[J]. Am J Physiol Heart Circ Physiol, 2011, 301(1): H12-H20. doi: 10.1152/ajpheart.00226.2011
|
| [13] |
Del Ry S, Cabiati M, Vozzi F, et al. Expression of C-type natriuretic peptide and its receptor NPR-B in cardiomyocytes[J]. Peptides, 2011, 32(8): 1713-1718. doi: 10.1016/j.peptides.2011.06.014
|
| [14] |
Ogawa T, de Bold AJ. The heart as an endocrine organ[J]. Endocr Connect, 2014, 3(2): R31-R44. doi: 10.1530/EC-14-0012
|
| [15] |
Sagnella GA. Measurement and significance of circulating natriuretic peptides in cardiovascular disease[J]. Clin Sci (Lond), 1998, 95(5): 519-529. doi: 10.1042/cs0950519
|
| [16] |
Rademaker MT, Richards AM. Cardiac natriuretic peptides for cardiac health[J]. Clin Sci (Lond), 2005, 108(1): 23-36. doi: 10.1042/CS20040253
|
| [17] |
Wei CM, Heublein DM, Perrella MA, et al. Natriuretic peptide system in human heart failure[J]. Circulation, 1993, 88(3): 1004-1009. doi: 10.1161/01.CIR.88.3.1004
|
| [18] |
Del Ry S, Passino C, Maltinti M, et al. C-type natriuretic peptide plasma levels increase in patients with chronic heart failure as a function of clinical severity[J]. Eur J Heart Fail, 2005, 7(7): 1145-1148. doi: 10.1016/j.ejheart.2004.12.009
|
| [19] |
Mangat H, de Bold AJ. Stretch-induced atrial natriuretic factor release utilizes a rapidly depleting pool of newly synthesized hormone[J]. Endocrinology, 1993, 133(3): 1398-1403. doi: 10.1210/endo.133.3.8365374
|
| [20] |
|
| [21] |
Gerzer R, Witzgall H, Tremblay J, et al. Rapid increase in plasma and urinary cyclic GMP after bolus injection of atrial natriuretic factor in man. J Clin Endocrinol Metab, 1985, 61(6): 1217-1219. doi: 10.1210/jcem-61-6-1217
|
| [22] |
Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins[J]. FASEB J, 1993, 7(2): 328-338. doi: 10.1096/fasebj.7.2.7680013
|
| [23] |
Melo LG, Steinhelper ME, Pang SC, et al. ANP in regulation of arterial pressure and fluid-electrolyte balance: lessons from genetic mouse models[J]. Physiol Genomics, 2000, 3(1): 45- 58. doi: 10.1152/physiolgenomics.2000.3.1.45
|
| [24] |
Vanderheyden M, Bartunek J, Goethals M. Brain and other natriuretic peptides: molecular aspects[J]. Eur J Heart Fail, 2004, 6(3): 261-268. doi: 10.1016/j.ejheart.2004.01.004
|
| [25] |
Wiley KE, Davenport AP. Physiological antagonism of endothelin-1 in human conductance and resistance coronary artery[J]. Br J Pharmacol, 2001, 133(4): 568-574. doi: 10.1038/sj.bjp.0704119
|
| [26] |
Furuya M, Yoshida M, Hayashi Y, et al. C-type natriuretic peptide is a growth inhibitor of rat vascular smooth muscle cells[J]. Biochem Biophys Res Commun, 1991, 177(3): 927-931. doi: 10.1016/0006-291X(91)90627-J
|
| [27] |
|
| [28] |
Brenner BM, Ballermann BJ, Gunning ME, et al. Diverse biological actions of atrial natriuretic peptide[J]. Physiol Rev, 1990, 70(3): 665-699. doi: 10.1152/physrev.1990.70.3.665
|
| [29] |
Burger AJ. A review of the renal and neurohormonal effects of B-type natriuretic peptide[J]. Congest Heart Fail, 2005, 11(1): 30-38. doi: 10.1111/chf.2005.11.issue-1
|
| [30] |
Sengenès C, Berlan M, De Glisezinski I, et al. Natriuretic peptides: a new lipolytic pathway in human adipocytes[J]. FASEB J, 2000, 14(10): 1345-1351. doi: 10.1096/fasebj.14.10.1345
|
| [31] |
Sengenes C, Stich V, Berlan M, et al. Increased lipolysis in adipose tissue and lipid mobilization to natriuretic peptides during low-calorie diet in obese women[J]. FASEB J, 2000, 14(10): 1345-1351. doi: 10.1096/fasebj.14.10.1345
|
| [32] |
Sengenès C, Zakaroff-Girard A, Moulin A, et al. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity[J]. Am J Physiol Regul Integr Comp Physiol, 2002, 283(1): R257-R265. doi: 10.1152/ajpregu.00453.2001
|
| [33] |
Sengenes C, Bouloumie A, Hauner H, et al. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone sensitive lipase phosphorylation in human adipocytes[J]. J Biol Chem, 2003, 278(49):48617-48626. doi: 10.1074/jbc.M303713200
|
| [34] |
|
| [35] |
Sarzani R, Marcucci P, Salvi F, et al. Angiotensin II stimulates and atrial natriuretic peptide inhibits human visceral adipocyte growth[J]. Int J Obes, 2008, 32(2): 259-267. doi: 10.1038/sj.ijo.0803724
|
| [36] |
Pierkes M, Gambaryan S, Bokník P, et al. Increased effects of C-type natriuretic peptide on cardiac ventricular contractility and relaxation in guanylyl cyclase A-deficient mice[J]. Cardiovasc Res, 2002, 53(4): 852-861. doi: 10.1016/S0008-6363(01)00543-0
|
| [37] |
Brusq JM, Mayoux E, Guigui L, et al. Effects of C-type natriuretic peptide on rat cardiac contractility[J]. Br J Pharmacol, 1999, 128(1): 206-212. doi: 10.1038/sj.bjp.0702766
|
| [38] |
Beaulieu P, Cardinal R, Pagé P, et al. Positive chronotropic and inotropic effects of C-type natriuretic peptide in dogs[J]. Am J Physiol, 1997, 273(4 Pt 2): H1933-H1940.
|
| [39] |
Tokudome T, Horio T, Soeki T, et al. Inhibitory effect of C-type natriuretic peptide (CNP) on cultured cardiac myocyte hypertrophy: interference between CNP and endothelin-1 signaling pathways[J]. Endocrinology, 2004, 145(5): 2131- 2140. doi: 10.1210/en.2003-1260
|
| [40] |
Santhekadur PK, Kumar DP, Seneshaw M, et al. The multifaceted role of natriuretic peptides in metabolic syndrome[J]. Biomed Pharmacother, 2017, 92: 826-835. doi: 10.1016/j.biopha.2017.05.136
|
| [41] |
Schlueter N, de Sterke A, Willmes DM, et al. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome[J]. Pharmacol Ther, 2014, 144(1): 12-27. doi: 10.1016/j.pharmthera.2014.04.007
|
| [42] |
|
| [43] |
Gruden G, Landi A, Bruno G. Natriuretic peptides, heart, and adipose tissue: new findings and future developments for diabetes research[J]. Diabetes Care, 2014, (11):2899-2908.
|
| [44] |
Wang D, Oparil S, Feng JA, et al. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse[J]. Hypertension, 2003, 42(1): 88-95. doi: 10.1161/01.HYP.0000074905.22908.A6
|
| [45] |
Vellaichamy E, Khurana ML, Fink J, et al. Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A[J]. J Biol Chem, 2005, 280(19): 19230-19242. doi: 10.1074/jbc.M411373200
|
| [46] |
Subramanian U, Kumar P, Mani I, et al. Retinoic acid and sodium butyrate suppress the cardiac expression of hypertrophic markers and proinflammatory mediators in Npr1 genedisrupted haplotype mice[J]. Physiol Genomics, 2016, 48(7): 477-490. doi: 10.1152/physiolgenomics.00073.2015
|
| [47] |
Sarzani R, Salvi F, Dessì-Fulgheri P, et al. Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans[J]. J Hypertens, 2008, 26(5): 831-843. doi: 10.1097/HJH.0b013e3282f624a0
|
| [48] |
Clerico A, Giannoni A, Vittorini S, et al. The paradox of low BNP levels in obesity[J]. Heart Fail Rev, 2012, 17(1): 81- 96. doi: 10.1007/s10741-011-9249-z
|
| [49] |
Moro C. Targeting cardiac natriuretic peptides in the therapy of diabetes and obesity[J]. Expert Opin Ther Targets, 2016, 20(12): 1445-1452. doi: 10.1080/14728222.2016.1254198
|
| [50] |
Baskin KK, Grueter CE, Kusminski CM, et al. MED13- dependent signaling from the heart confers leanness by enhancing metabolism in adipose tissue and liver[J]. EMBO Mol Med, 2014, 6(12): 1610-1621. doi: 10.15252/emmm.201404218
|
| [51] |
Lee JH, Bassel-Duby R, Olson EN. Heart- and muscle-derived signaling system dependent on MED13 and Wingless controls obesity in Drosophila[J]. Proc Natl Acad Sci USA, 2014, 111(26): 9491-9496. doi: 10.1073/pnas.1409427111
|
| [52] |
Konzer A, Ruhs A, Braun T, et al. Global protein quantification of mouse heart tissue based on the SILAC mouse[J]. Methods Mol Biol, 2013, 1005: 39-52. doi: 10.1007/978-1-62703-386-2
|
| [53] |
|
| [54] |
Gioia M, Foster LJ, Overall CM. Cell-based identification of natural substrates and cleavage sites for extracellular proteases by SILAC proteomics[J]. Methods Mol Biol, 2009, 539: 131- 153. doi: 10.1007/978-1-60327-003-8
|
| [55] |
Ong SE, Blagoev B, Kratchmarova I, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics[J]. Mol Cell Proteomics, 2002, 1(5): 376-386. doi: 10.1074/mcp.M200025-MCP200
|
| [56] |
Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation[J]. Nat Immunol, 2001, 2(2): 102-107. doi: 10.1038/84205
|
| [57] |
|
| [58] |
Steinberg GR, Michell BJ, van Denderen BJ, et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling[J]. Cell Metab, 2006, 4(6): 465-474. doi: 10.1016/j.cmet.2006.11.005
|
| [59] |
Tse MCL, Herlea-Pana O, Brobst D, et al. Tumor necrosis factor-alpha promotes phosphoinositide 3-kinase enhancer A and AMP-activated protein kinase interaction to suppress lipid oxidation in skeletal muscle[J]. Diabetes, 2017, 66(7): 1858- 1870. doi: 10.2337/db16-0270
|


 Authors and Reviewers
Authors and Reviewers

 DownLoad:
DownLoad: