| Citation: | Lei Xu, Ran Li, Juan Li, Zhou Dong, Jiaxin Zong, Chuchu Tan, Zekang Ye, Lu Shi, Xiaoxuan Gong, Chunjian Li. Simultaneous determination of clopidogrel, 2-oxo-clopidogrel, and the thiol metabolite of clopidogrel in human plasma by LC-MS/MS[J]. The Journal of Biomedical Research, 2022, 36(2): 109-119. DOI: 10.7555/JBR.36.20210125 |
Clopidogrel, (+)-(S)-methyl 2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridine-5(4H)-yl)acetate, is an anti-platelet agent. It has been extensively used to prevent ischemic stroke, myocardial infarction, and cardiovascular diseases[1]. However, its pharmacodynamics differ remarkably among patients[2], which is considered to be associated with the unique pharmacokinetics of clopidogrel.
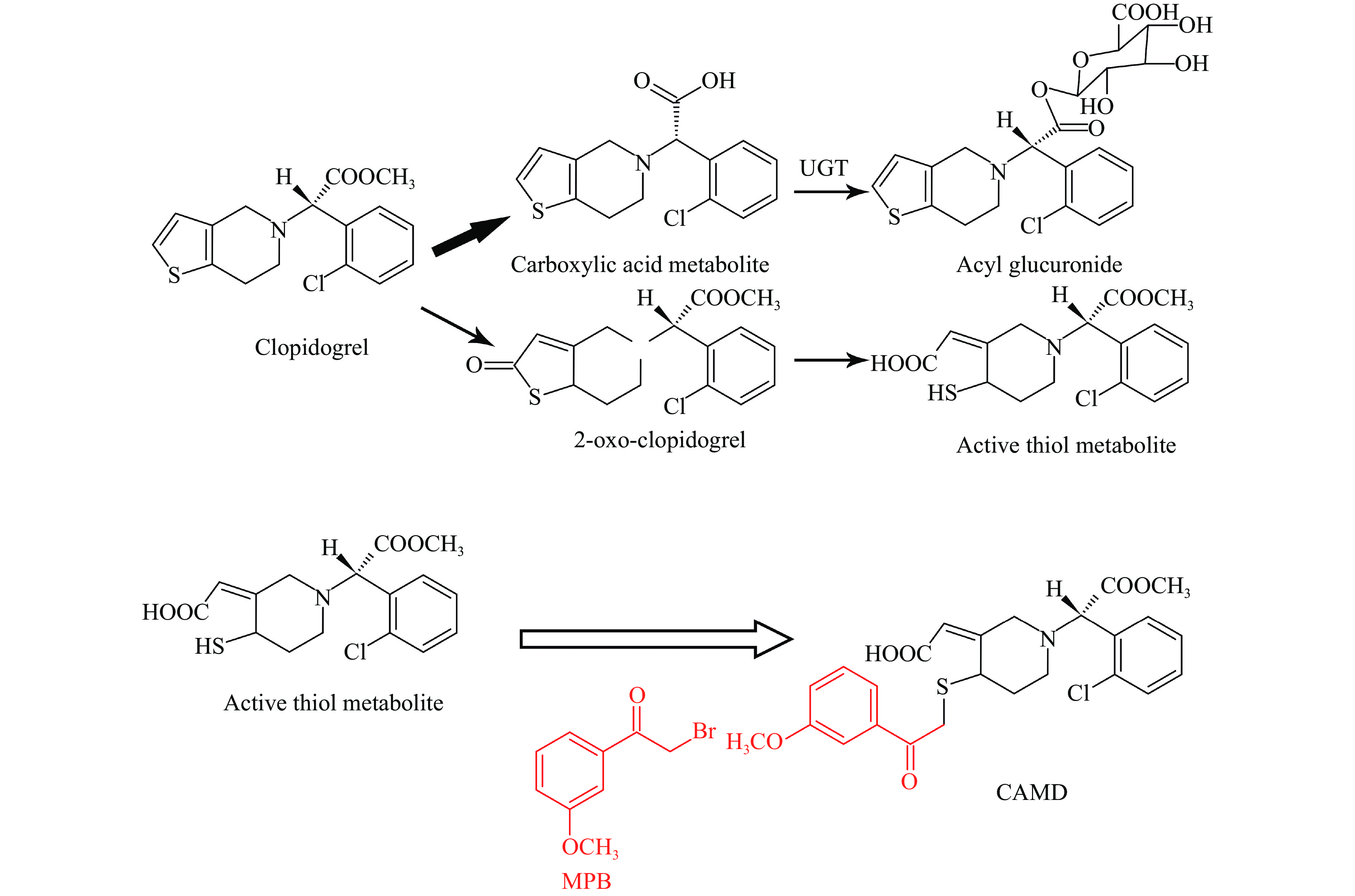
Clopidogrel is an orally bioavailable pro-drug, which (approximately 85%) is hydrolyzed into inactive metabolites by carboxylesterases, and then partially converted to the corresponding acyl glucuronide[3–4]. Additionally, a portion of the remained clopidogrel is metabolized into its thiolactone intermediate, 2-oxo-clopidogrel (2-Oxo-CLP). 2-Oxo-CLP is further hydrolyzed by thioester, cleaving the thiolactone ring and finally generating the active thiol metabolite (Fig. 1). Functionally, the active thiol metabolite binds to P2Y12 receptor and irreversibly inhibits ADP-induced platelet aggregation[5]. The hepatic cytochrome P450 family 2 subfamily C member 19 genotypes (CYP2C19) contributes to the two steps of clopidogrel metabolism by 45% and 20%, respectively, in which clopidogrel is metabolized to 2-Oxo-CLP and active metabolite[6].
It has been reported that approximately 20% to 30% of patients with coronary artery disease (CAD) have low or no response to clopidogrel due to the CYP2C19 gene variation and consequent less production of the active clopidogrel, which leads to increased clinical events, such as stent thrombosis and myocardial infarction[7–10]. However, the Gauging Responsiveness with A VerifyNow Assay-Impact on Thrombosis and Safety (GRAVITAS) trial showed that the platelet function test (PFT)-guided anti-platelet treatment did not reduce the major adverse clinical events[11]. Based on this, the pharmacodynamic tests are not recommended for guiding the anti-platelet therapy (Recommendation: Class Ⅲ; Level of Evidence: A)[12]. Our previous study showed a moderate correlation between platelet aggregation and clopidogrel active metabolite (CAM) levels, suggesting that PFT may not be suitable for measuring clopidogrel therapy adequacy. The pharmacokinetics of clopidogrel should be investigated to clarify the phenomenon of low or no response to clopidogrel, and the efficacies of pharmacokinetic parameters, e.g., concentrations of clopidogrel, 2-Oxo-CLP, and CAM, might be useful to guide the anti-platelet treatment[13].
Previous studies have reported different methods which used liquid chromatography-tandem mass spectrometry (LC-MS/MS) for quantification of clopidogrel or its metabolites. Peer et al quantified clopidogrel and cis-clopidogrel-MP derivative from two separated plasma samples by using a sensitive and rapid ultra HPLC-MS/MS strategy[14]. Karaźniewicz-Łada et al simultaneously determined the concentrations of clopidogrel, its carboxylic acid metabolite and the derivative isomers of thiol metabolite by using a triple-quadrupole MS with multiple-reaction-monitoring via electrospray ionization[15]. Silvestro et al simultaneously quantified clopidogrel, clopidogrel carboxylic acid and the newly described acyl glucuronide metabolite[16]. However, there is still a lack of functional methods to simultaneously quantify clopidogrel, 2-Oxo-CLP, and CAM, especially for the patients with CAD.
In the present study, a high-throughput LC-MS/MS method was developed to simultaneously measure the concentrations of clopidogrel, 2-Oxo-CLP, and CAM. Meanwhile, this method was validated in 3 CAD patients undergone stent implantation.
Clopidogrel hydrogensulfate (purity ≥98%) was purchased from Sigma-Aldrich Chemie (Germany). 2-Oxo-CLP hydrochloride and clopidogrel active metabolite derivative (CAMD) were obtained from Toronto Research Chemicals Inc. (Canada). The alkylating agent 2-bromo-3'-methoxyacetophenome (MPB) was bought from Sigma-Aldrich Chemie. Mifepristone (internal standard, IS) was supplied by Xianju Pharmaceutical Co., Ltd. (China). 1,4-Dithio-DL-threitol (DTT, purity ≥98%) and ascorbic acid (purity ≥99%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (China). Acetonitrile and methyl tert-butyl ether (MTBE) of HPLC/Spectro grade were obtained from Tedia Company Inc. (USA). Other chemicals were all of analytical grade and purchased from Nanjing Chemical Reagent Co., Ltd. (China). Blank plasma was obtained from the Nanjing Branch, Red Cross Society of China.
Stock solutions of clopidogrel, 2-Oxo-CLP, CAMD, and IS were prepared individually in acetonitrile with the following concentrations: 10 μg/mL for clopidogrel, 10 μg/mL for 2-Oxo-CLP, 10 μg/mL for CAMD, and 1 mg/mL for IS. After vortexing and brief sonication, all the stock solutions were stored at −20 °C. For plasma sample analysis, the standard solutions of clopidogrel, 2-Oxo-CLP, CAMD were prepared from stock solutions by diluting the appropriate volume of the stock solution with anhydrous acetonitrile in 10 mL glass flasks. The standard solutions for clopidogrel were at the doses of 0.5, 1, 2, 8, 100, 200, 300, 400, and 500 ng/mL. For the 2-Oxo-CLP, the doses were 5, 10, 20, 80, 100, 200, 300, 400, and 500 ng/mL. For the CAMD, the doses were 5, 10, 20, 80, 200, 400, 600, 800, and 1000 ng/mL, and for the IS, the dose was 10 μg/mL. Working dilutions were freshly prepared from stock solutions of the analytes or IS when needed. Calibration standards were prepared by spiking 10 μL standard solutions into 90 μL human plasma, and they were 0.05, 0.1, 0.2, 0.8, 10, 20, 30, 40, and 50 ng/mL for clopidogrel, 0.5, 1, 2, 8, 10, 20, 30, 40, and 50 ng/mL for 2-Oxo-CLP, 0.5, 1, 2, 8, 20, 40, 60, 80, and 100 ng/mL for CAMD. Quality control (QC) samples were at three different concentrations (0.1, 20, and 40 ng/mL for clopidogrel, 1, 20, and 40 ng/mL for 2-Oxo-CLP, and 1, 40, and 80 ng/mL for CAMD).
Firstly, 400 μL blank samples were mixed with 40 μL calibration standard and 40 μL QC samples in 10 mL glass tubes containing 50 μL DTT (20 mmol/L). While detecting the patients' samples, the QC samples were replaced by equal volume of anhydrous acetonitrile in order to guarantee the equivalent testing system. Then, 100 μL hydrochloric acid (0.05 mol/L) was added and vortexed for 10 seconds. Next, 1.5 mL MTBE was added and vortexed for 3 minutes. The solution was centrifuged at 2000 g for 10 minutes and an aliquot of 1.2 mL supernatant was transferred into a new test tube, then 50 μL DTT (20 mmol/L) was further added, and the supernatant was evaporated to dryness under vacuum at 40 °C. The residue was reconstituted in a 150 μL mobile phase containing 0.1% aqueous formic acid and acetonitrile (1:19, v/v) and centrifuged at 16 000 g for 10 minutes. Finally, 30 μL aliquot was injected into the LC-MS/MS system.
LC-MS/MS was performed using a Waters Alliance 2695 LC system accompanied with a column oven (Milford, USA) and coupled with a Micromass Quattro Micro tandem MS system (Micromass, UK), which was equipped with an electrospray ionization source and operated with the MassLynx 4.0 software.
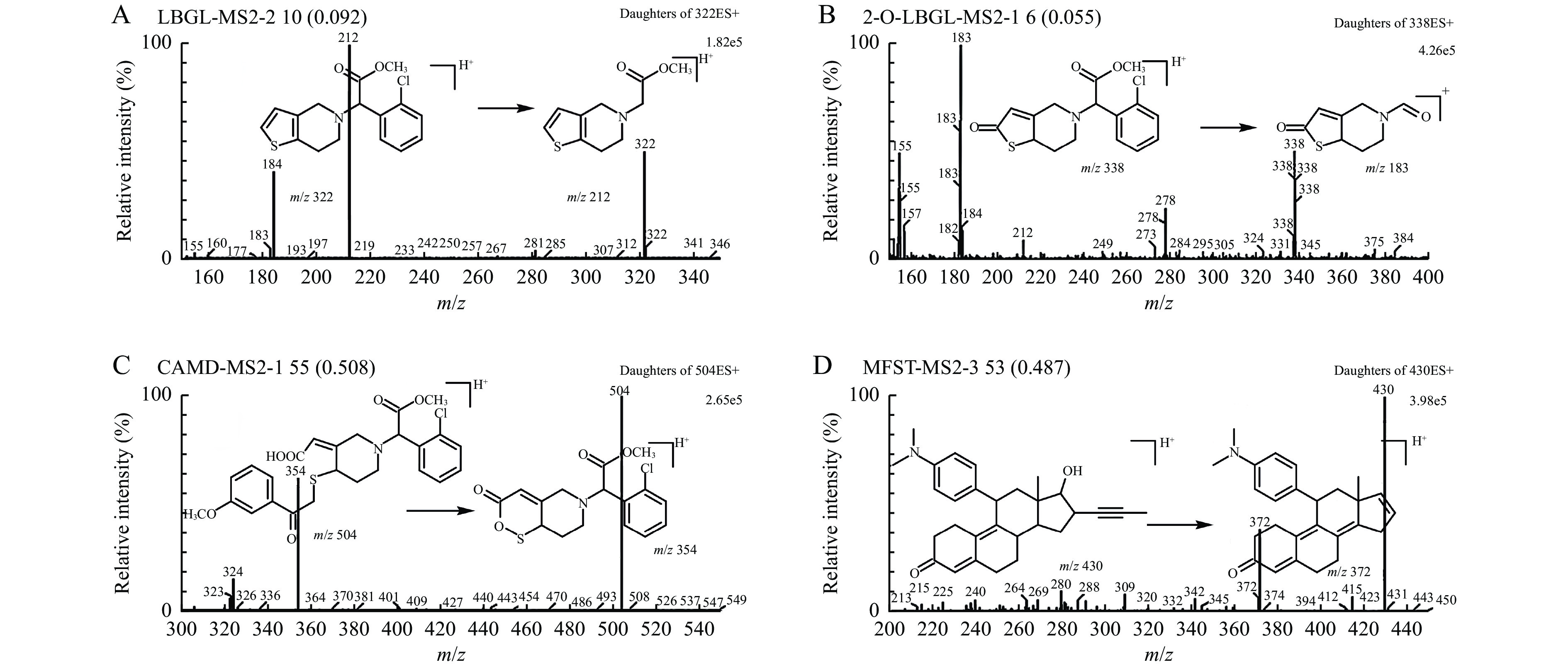
The analytes were separated on a Sapphire C18 analytical column (250 mm × 4.6 mm, 5 μm; Sepax Technologies Inc., USA) with a Security Guard C18 guard column (25 mm × 3.0 mm, 5 μm; Phenomenex, USA), which were maintained at 40 °C using a mobile phase of 0.1% aqueous formic acid and acetonitrile (1:19, v/v) at a flow rate of 1 mL/min. The volume of injection was 30 μL and the analytical run time was 5.5 minutes. The eluent from the HPLC column was introduced directly to the micromass using the electrospray ionization interface in the positive ion mode. The detection parameters were optimized as follows: source temperature, 120 °C; nitrogen desolvation gas, 370 °C with a flow rate of 500 L/hours. The specific transitions for the analytes were monitored using multiple-reaction-monitoring mode. The transitions, m/z 322.0 → 212.0 for clopidogrel; m/z 338.0 → 183.0 for 2-Oxo-CLP; m/z 504.0 → 354.0 for CAMD; and m/z 430.0 → 372.0 for IS, were chosen for the quantification of the analytes.
The LC-MS/MS method was validated by the Food and Drug Administration (FDA) guidelines for bioanalytical method validation.
Linearity of the calibration curves was assessed in duplicate on three consecutive days. A series of working solutions were obtained by gradient dilution. The standard curves were established ranging from 0.05 to 50 ng/mL (0.05, 0.1, 0.2, 0.8, 10, 20, 30, 40, and 50 ng/mL) for clopidogrel, 0.5 to 50 ng/mL (0.5, 1, 2, 8, 10, 20, 30, 40, and 50 ng/mL) for 2-Oxo-CLP, and 0.5 to 100 ng/mL (0.5, 1, 2, 8, 20, 40, 60, 80, and 100 ng/mL) for CAMD. The peak area of the analytes was used for quantification by using 1/x2 as a weighting factor (where x was the concentration of the analyte). The correlation coefficient of the calibration curve was determined and the equations of calibration curves were used to calculate the concentrations of clopidogrel, 2-Oxo-CLP and CAMD in patients' plasma.
Lower limit of quantification (LLOQ) was defined as the lowest concentration of analytes. The intra- and inter-day accuracies, expressed as relative error (RE), were calculated by the formula: RE=(nominal sample concentration−tested sample concentration)/nominal sample concentration×100%. The intra- and inter-day precisions, expressed as relative standard deviation (RSD), were calculated by the formula: RSD=SD value/mean value×100%.
The QC samples were prepared in six replicates analyzed over three different days at plasma concentrations of 0.1 ng/mL, 20 ng/mL, and 40 ng/mL for clopidogrel, 1.0 ng/mL, 20 ng/mL, and 40 ng/mL for 2-Oxo-CLP and 1.0 ng/mL, 40 ng/mL, and 80 ng/mL for CAMD.
Stabilities of plasma clopidogrel, 2-Oxo-CLP and CAMD were evaluated at concentrations of 0.1 and 10 ng/mL for clopidogrel, 2.0 and 50 ng/mL for 2-Oxo-CLP, and 2.0 and 50 ng/mL for CAMD (three replicates for each analytes concentration) after three freeze-thaw cycles, short-term storage (plasma standing at room temperature for 8 hours; extract standing at room temperature for 8 hours), and long-term storage (45 days at −80 °C). Moreover, the stability of the processed samples were examined after being stored at 4 °C for 24 hours, and the stability of the analytes were examined after being stored at −20 °C for 2 months in stock solutions. Considering the potential degradation of stock solution, we prepared and used it within one week. The independent experiments were performed in three replicates.
The extraction efficiency was evaluated by using plasma samples of the calibration curve at concentrations of 0.1, 20, and 40 ng/mL for clopidogrel, 1.0, 20, and 40 ng/mL for 2-Oxo-CLP and 1.0, 40, and 80 ng/mL for CAMD, respectively. The reference samples of extraction efficiency were prepared by adding clopidogrel, 2-Oxo-CLP, CAMD and IS working solutions to blank plasma extracts. The recovery was acceptable if the coefficient of variation (CV) was within ±20%. To investigate the matrix effect (ME), the reference samples of extraction efficiency were compared with reference solutions without matrix. The CV value of ME was acceptable if it was within 15%.
The validated LC-MS/MS method was demonstrated for quantitative determination of clopidogrel, 2-Oxo-CLP, and CAMD in the CAD patients' plasma. This study was conducted in compliance with the revised Declaration of Helsinki for biomedical research involving human subjects and the rules of good clinical practice (GCP), and was approved by Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Permit No. 2011-SRFA-099). Informed consent was obtained from all subjects before the study initiation.
Three patients with CAD and stent implantation were enrolled after taking oral Plavix (Sanofi [Hangzhou] Pharmaceuticals Co., Ltd., China), containing 75 mg clopidogrel, for more than 5 days. The Plavix tablet was required to be taken with 250 mL water within half an hour after breakfast. Three milliliters of venous blood was collected into tubes containing EDTA and DTT before Plavix administration and 0.5, 1, 2, 2.5, 3, 3.5, 6, 8, 12, and 24 hours after administration, and 30.0 μL of MPB (500 mmol/L) was added in the blood samples immediately after collection. Then the samples were separated by centrifugation at 16 000 g for 10 minutes and frozen at −80 °C until assayed for clopidogrel, 2-Oxo-CLP and CAMD.
Statistical analysis was performed by using SPSS 26.0 software (IBM, USA). The data are presented as mean±standard deviation (SD) for continuous variables.
The MS conditions were optimized for obtaining an appropriate resolution and high sensitivity of clopidogrel, 2-Oxo-CLP, CAMD, and IS in the plasma samples. The transitions, m/z 322.0 → 212.0 for clopidogrel, m/z 338.0 → 183.0 for 2-Oxo-CLP, m/z 504.0 → 354.0 for CAMD, and m/z 430.0 → 372.0 for IS, were chosen for quantification of analytes. MS collision parameters for each compound are listed in Table 1. The full scan product ion spectra and proposed fragmentation pattern of analytes and IS are shown in Fig. 2.
| Analytes | Precursor ion (m/z) | Fragment ion (m/z) | Cone voltage (V) | Collision energy (eV) |
| Clopidogrel | 322.0 | 212.0 | 22 | 15 |
| 2-Oxo-CLP | 338.0 | 183.0 | 24 | 17 |
| CAMD | 504.0 | 354.0 | 27 | 19 |
| IS | 430.0 | 372.0 | 28 | 21 |
| 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative; IS: internal standard. | ||||
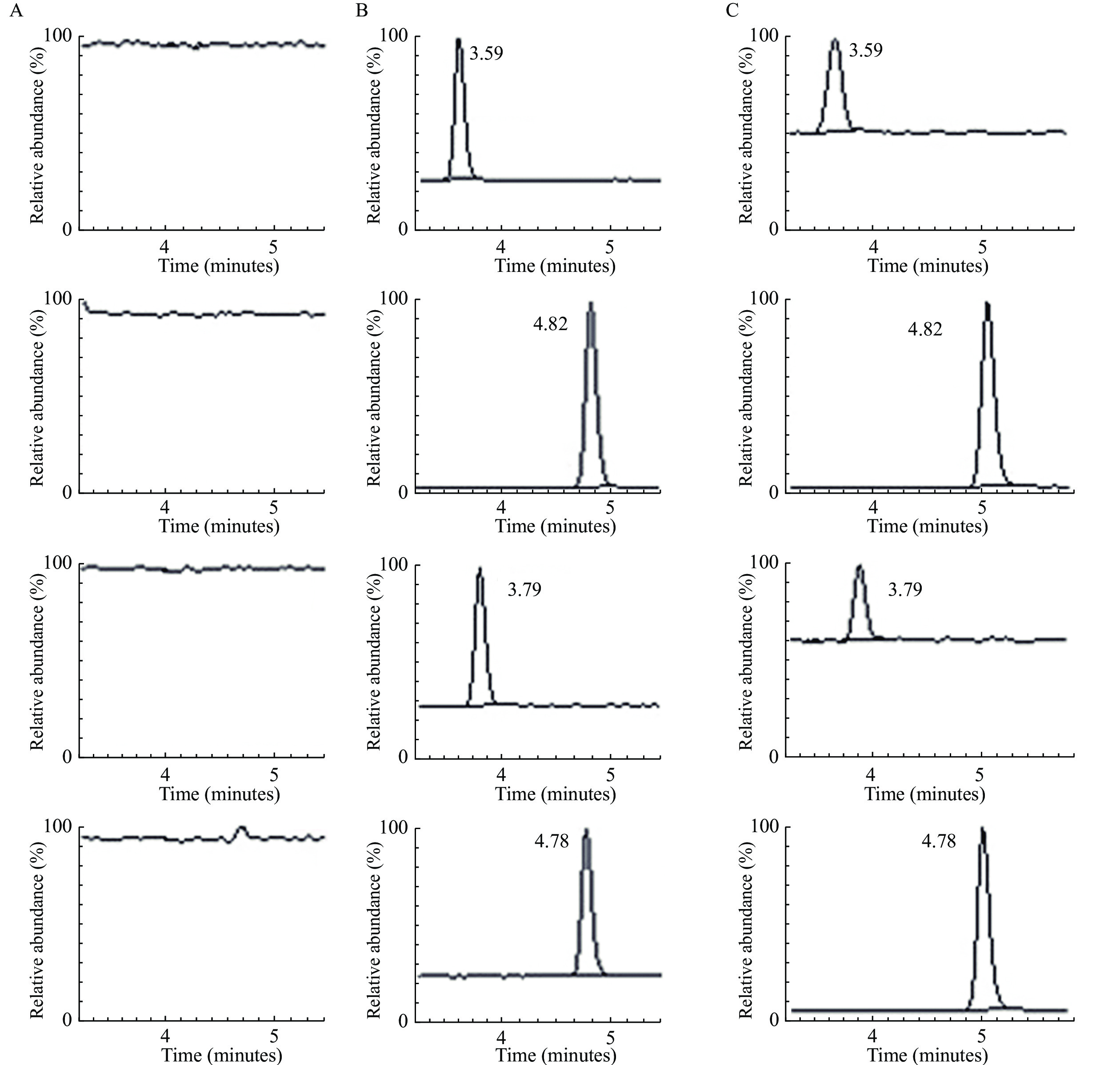
It is important to optimize chromatographic conditions, such as analytical column and mobile phase, to achieve an adequate retention of analytes on column and desired separation with endogenous matrix. In addition, clopidogrel acyl glucuronide is confirmed as a major source of back-conversion to clopidogrel in the presence of methanol. Since transesterification did not occur in acetonitrile, acetonitrile was chosen as the organic additive in mobile phase. The usage of 0.1% aqueous formic acid in water resulted in good retention and peak shapes without any ion suppression, and the retention time (tR) of clopidogrel, 2-Oxo-CLP, CAMD, and IS was 4.78, 3.79, 3.59, and 4.82 minutes, respectively, with a short run time of 5.5 minutes, which was important for this high-throughput analysis.
As an active metabolite, the thiol metabolite of clopidogrel was unstable in plasma because of the reactivity of the thiol group, which is prone to form a disulfide bond with either itself, or endogenous low molecular-weight compounds, or plasma proteins. To prevent these reactions and stabilize the thiol metabolite, MPB was selected as an alkylating reagent blocker[17]. The derivatization of clopidogrel active metabolite with MPB yielded the compound of CAMD, the chemical structures of which are shown in Fig. 1. MPB was initially believed to have a deleterious effect on clopidogrel signals in the mass spectrometer. However, it did not affect the qualification of clopidogrel, neither did it cause any significant MEs[16]. Therefore, the active metabolite of clopidogrel was immediately derived along with the blood sample collection, and thus ensuring its stability during sample handling and storage for the simultaneous qualification.
2-Oxo-CLP has the tendency to be oxidized to the sulfoxide or sulfone without a reducing agent. DTT, ascorbic acid and other reducing agents can be used to maintain 2-Oxo-CLP in an un-oxidized state during sample collection, preparation and analysis. Hence, we investigated the impact of ascorbic acid and DTT on the experimental results. It was found that compared with DTT, the sensitivity of the three analytes was lower when choosing the ascorbic acid, because of the solubility of ascorbic acid. Consequently, to get better stability and extraction of 2-Oxo-CLP, 50 μL of DTT (20 mmol/L) was added into the supernatant, which was consolidated by analyzing supernatant with or without DTT. As shown in Table 2, great variation of the recovery results could be observed without DTT.
| Analytes | Concentration (ng/mL) | Recovery | Matrix effect | |||
| (%) | RSD (%) | (%) | RSD(%) | |||
| Clopidogrel | 0.1 | 101.9±8.3 | 8.1 | 76.7±2.3 | 3.0 | |
| 40 | 81.5±12.0 | 14.7 | 75.8±10.0 | 13.2 | ||
| 2-Oxo-CLP | 1 | 70.2±22.1 | 31.5 | 74.6±9.7 | 13.0 | |
| 40 | 62.4±6.1 | 9.7 | 60.3±0.5 | 0.9 | ||
| CAMD | 1 | 72.2±26.9 | 37.2 | 75.1±9.7 | 12.9 | |
| 80 | 82.4±15.2 | 18.4 | 69.8±2.2 | 3.1 | ||
| All the data are presented as mean±SD. 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative; DTT: 1, 4-Dithio-DL-threitol; RSD: relative standard deviation. | ||||||
The impacts of HCl concentrations on extraction efficiencies and MEs of the three analytes have been studied. Extraction efficiency of the active metabolite was extremely low (about 30%) when HCl (0.01 mol/L) was added, because carboxylic acid group of the active metabolite could be dissociated into carboxylate anion. In contrast, the matrix suppression effects of the three analytes were significant in presence of HCl (0.1 mol/L). It's worth noting that 0.05 mol/L HCl provided better extraction efficiency and MEs for the three analytes (Table 3). We chose mifepristone as IS due to its similar polarity and stable ionization rate to clopidogrel as well as its metabolites without interference[18].
| Concentration (ng/mL) | 0.01 mol/L HCl | 0.05 mol/L HCl | 0.1 mol/L HCl | ||||||
| Extraction efficiency (%) | Matrix effect (%) | Extraction efficiency (%) | Matrix effect (%) | Extraction efficiency (%) | Matrix effect (%) | ||||
| Clopidogrel | 0.1 | 170.9±8.9 | 364.0±10.2 | 107.4±6.2 | 75.0±2.8 | 194.4±9.8 | 52.9±2.9 | ||
| 20 | 147.5±6.1 | 107.4±4.5 | 116.5±2.2 | 94.1±10.0 | 155.7±7.4 | 52.1±4.0 | |||
| 40 | 138.8±5.4 | 88.2±4.3 | 118.7±8.7 | 71.9±5.9 | 134.0±6.1 | 49.0±3.1 | |||
| 2-Oxo-CLP | 1 | 96.5±6.4 | 72.1±4.3 | 92.5±3.9 | 63.7±3.4 | 115.1±9.5 | 40.5±3.6 | ||
| 20 | 103.2±3.4 | 61.3±4.0 | 117.0±3.5 | 71.6±5.0 | 148.3±6.5 | 38.2±3.1 | |||
| 40 | 103.9±3.2 | 64.5±4.3 | 110.9±7.8 | 63.7±4.6 | 133.7±3.5 | 39.5±2.0 | |||
| CAMD | 1 | 32.1±2.3 | 84.0±2.5 | 96.7±3.2 | 70.8 ±5.3 | 93.4±13.3 | 52.2±6.3 | ||
| 40 | 34.8±1.6 | 80.7±3.8 | 123.4±3.9 | 53.0±5.8 | 171.6±5.3 | 39.7±2.1 | |||
| 80 | 34.0±2.6 | 88.4±2.3 | 120.2±7.0 | 59.3±5.0 | 167.9±5.1 | 53.8±4.6 | |||
| All the data were presented as mean±SD. 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative. | |||||||||
Independent LLOQ experiments were performed by preparing the LLOQ in 6 different lots of the plasma, and back-calculating the concentration as a 'QC' sample. The concentrations of LLOQ were 0.05 ng/mL for clopidogrel, 0.50 ng/mL for 2-Oxo-CLP, and 0.50 ng/mL for CAMD, respectively. The mean accuracy (RE from the nominal standard) of clopidogrel, 2-Oxo-CLP, and CAMD was 13.3%, 10.4%, and 7.7%, respectively. The RSD were 13.2%, 5.1%, and 1.9%, respectively. These results showed that the concentrations of LLOQ for clopidogrel, 2-Oxo-CLP, and CAMD could be detected precisely.
Typical chromatograms of extracts from the LC-MS/MS analysis are presented in Fig. 3. The tR values for clopidogrel (1.00 ng/mL), 2-Oxo-CLP (10.0 ng/mL), CAMD (10.0 ng/mL), and IS (10.0 μg/mL) were 4.78 minutes, 3.79 minutes, 3.59 minutes, and 4.82 minutes, respectively. Similar results were observed in the plasma sample collected from a CAD patient 2 hours after taking clopidogrel for 75 mg. Furthermore, no carryover was observed for either analyte or IS by running a blank sample following the upper limit of quantification (ULOQ; 50 ng/mL for clopidogrel, 50 ng/mL for 2-Oxo-CLP, 100 ng/mL for CAMD), and no analyte or IS peak was detected at their respective retention time. The results indicated that no endogenous interferences existed while detecting these analytes.
Standard curves estimated for the analytes showed good linearity over the concentration ranges as follows: 0.05 to 50.0 ng/mL for clopidogrel, 0.5 to 50.0 ng/mL for 2-Oxo-CLP, and 0.5 to 100.0 ng/mL for CAMD. The equations of standard curves and correlation coefficients are presented in Table 4.
| Compound (ng/mL) | Calibration curve equations | Correlation coefficient (r) |
| Clopidogrel (0.05–50.0) | Pclopidogrel/PIS=0.08147×Cclopidogrel +0.001820 | 0.9984 |
| 2-Oxo-CLP (0.5–50.0) | P2-OXO-CLP/PIS=0.009094×C2-Oxo-CLP +0.0003292 | 0.9974 |
| CAMD (0.5–100.0) | PCAMD/PIS=0.01072×CCAMD+0.0004895 | 0.9979 |
| 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative. | ||
Intra- and inter-day accuracies of the method were expressed as RE by pointing to relatively high accuracy in the estimation of the investigated analytes' concentrations in plasma samples. Intra- and inter-day precisions of the method were expressed as RSD by fitting the ranges that were required for testing drug and/or metabolite's contents in body fluids (Table 5). The FDA guideline suggests that the variability is allowed within ranges of ±15.0% for RSD, except for the LLOQ, where ±20% variability is acceptable. These results proved that our method was accurate and precise in per se.
| Analyte | Nominal concentration (ng/mL) | Intra-day (n=6) | Inter-day (n=3) | |||||
| Mean assayed value (ng/mL) | Accuracy (RE [%]) | Precision (RSD [%]) | Mean assayed value (ng/mL) | Accuracy (RE [%]) | Precision (RSD [%]) | |||
| Clopidogrel | 0.1 | 0.1 | 15.2 | 4.8 | 0.1 | 12.2 | 18.7 | |
| 20 | 20.7 | 3.6 | 2.2 | 20.4 | 2.0 | 3.7 | ||
| 40 | 41.8 | 4.5 | 2.7 | 42.4 | 5.9 | 5.6 | ||
| 2-Oxo-CLP | 1 | 1.1 | 5.8 | 4.7 | 1.0 | 2.8 | 19.2 | |
| 20 | 21.2 | 5.8 | 4.0 | 20.6 | 2.9 | 6.4 | ||
| 40 | 41.0 | 2.7 | 5.8 | 42.4 | 6.0 | 7.7 | ||
| CAMD | 1 | 1.0 | 2.8 | 8.5 | 1.0 | 4.0 | 14.8 | |
| 40 | 42.0 | 5.0 | 4.3 | 42.0 | 5.1 | 8.8 | ||
| 80 | 83.0 | 3.7 | 7.2 | 84.6 | 5.8 | 5.9 | ||
| 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative; RE: relative error, RE=(nominal sample concentration–tested sample concentration)/nominal sample concentration×100%; RSD: relative standard deviation, RSD=SD value/mean value×100%. | ||||||||
DTT and MPB were added to the samples for the stability of 2-Oxo-CLP and CAM. Stock solutions of clopidogrel, 2-Oxo-CLP, CAMD, and IS in acetonitrile were stable at −20 °C for at least 2 months. In human plasma, no significant degradation (within ±15% deviation between the predicted and nominal concentrations) of clopidogrel, 2-Oxo-CLP, and CAMD occurred under different storage conditions. The stability test results are summarized in Table 6.
| Analytes | Added (ng/mL) | Found (ng/mL) | ||||
| At –80 °C for 45 days | After three-thaw cycles | Plasma at room temperature for 8 hours | Extract at room temperature for 8 hours | Extract in the auto-sampler at 4 °C for 24 hours | ||
| Clopidogrel | 0.2 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 |
| 10 | 10.1±0.2 | 10.1±0.8 | 10.4±0.8 | 10.0±1.4 | 10.0±0.8 | |
| 2-Oxo-CLP | 2 | 2.0±0.1 | 1.9±0.2 | 2.2±0.4 | 2.1±0.2 | 2.2±0.2 |
| 50 | 50.6±1.2 | 51.0 ± 3.9 | 52.2±5.1 | 50.1±7.6 | 49.0±3.7 | |
| CAMD | 2 | 2.0±0.1 | 1.9 ± 0.2 | 2.2±0.4 | 2.0±0.2 | 2.1±0.2 |
| 50 | 50.4±1.2 | 51.5±3.8 | 51.1±4.9 | 49.6±7.7 | 50.1±3.9 | |
| All the data are presented as mean±SD. 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative. | ||||||
The extraction recoveries in human plasma were (107.4±6.2)%, (116.5±2.2)%, and (118.7±8.7)% at 0.1, 20, and 40 ng/mL for clopidogrel, (92.5±3.9)%, (117.0±3.5)%, and (110.9±7.8)% at 1, 20, and 40 ng/mL for 2-Oxo-CLP, and (96.7±3.2)%, (123.4±3.9)%, and (120.2±7.0)% at 1, 40, and 80 ng/mL for CAMD, respectively. For the IS, the extraction recovery at an initial concentration of 10 μg/mL was (111.4±8.2)%. The ME of this study was evaluated by analyzing samples at three concentration levels (0.1, 20, and 40 ng/mL for clopidogrel, 1, 20, and 40 ng/mL for 2-Oxo-CLP, and 1, 40, and 80 ng/mL for CAMD). The mean MEs of the six different samples were 75.0%, 94.1%, and 71.9% for clopidogrel, 63.7%, 71.6%, and 63.7% for 2-Oxo-CLP, and 70.8%, 53.0%, and 59.3% for CAMD at the three concentration levels. The mean ME of IS was 77.0%. The CV values of MEs were all within 15% (2.5%, 10.6%, and 8.2% for different doses of clopidogrel, 5.4%, 7.0%, and 7.3% for different doses of 2-Oxo-CLP, and 7.4%, 11.0%, and 8.5% for different doses of CAMD), indicating that the impact of the extracted plasma matrix was negligible and consistent. The extraction recovery and ME for clopidogrel, 2-Oxo-CLP, and CAMD were consistent when examined repeatedly at the same concentration or at variant concentrations (Table 3).
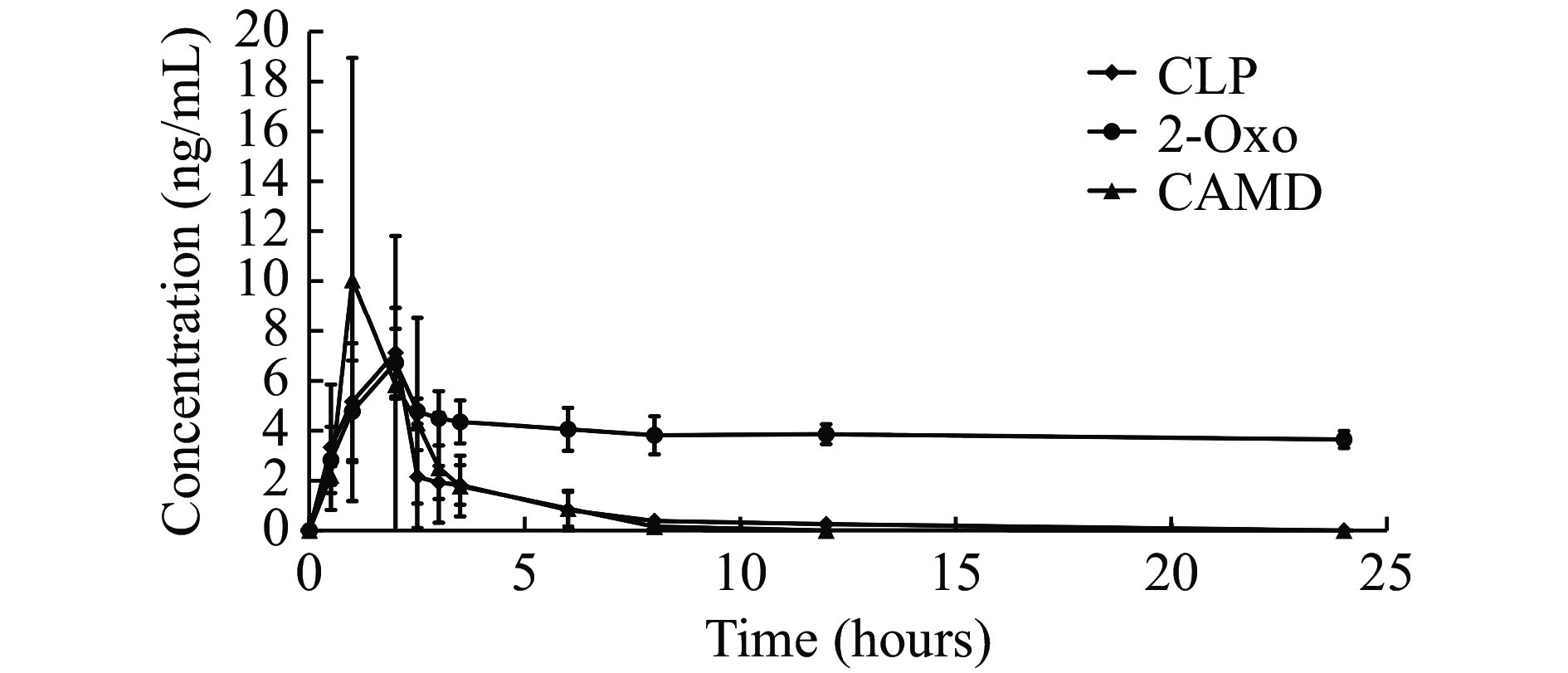
The utility of the assay for pharmacokinetic studies has been demonstrated in CAD patients following a maintenance dosing of 75 mg daily clopidogrel. The mean plasma concentration-time profiles of clopidogrel, 2-Oxo-CLP, and CAMD are presented in Fig. 4, which shows a typical pharmacokinetic profile obtained for clopidogrel, 2-Oxo-CLP, and CAMD. With one CAD patient reaching a maximum plasma concentration (Cmax) at 17.0 ng/mL, the clopidogrel calibration range (0.05 to 50 ng/mL) was sufficient to measure its plasma levels observed in the clinical settings. The same method was applied to the 2-Oxo-CLP calibration range (0.5 to 50 ng/mL), and the plasma levels of 2-Oxo-CLP in the patient also reached a Cmax at 9 ng/mL. For the CAMD calibration, the Cmax was at 16.0 ng/mL, which was involved in the CAMD calibration range (0.5 to 100 ng/mL).
Interestingly, 24 hours after drug administration, we found that the concentration of 2-Oxo-CLP remained at a high level. This phenomenon may be caused by complex metabolic pathways of 2-Oxo-CLP, since multiple metabolic pathways along with the active metabolite formation of 2-Oxo-CLP existed[19]. The levels of CAMD reached their peaks before those of clopidogrel. Such a phenomenon could be explained by the back conversion problem or a rapid in-vivo degradation of this active clopidogrel metabolite. The bona fide reason remains to be quenched in future investigations.
Our study has successfully developed an LC-MS/MS method which allow simultaneous determination of clopidogrel, 2-Oxo-CLP, and the active thiol metabolite of clopidogrel.
CAD patients who were under clopidogrel treatment and present with thrombosis or bleeding events need adjustment of the dosage or selection of proper P2Y12 inhibitor. However, the platelet function tests are not recommended to guide the anti-platelet treatment in this scenario[12]. Thus, it is important to investigate the pharmacokinetics of clopidogrel for the guidance on the clinical treatment, and to develop an effective and applicable method to determine clopidogrel as well as its essential metabolites.
Previous studies have reported the determination of clopidogrel as well as its metabolites[15,20]. However, to the best of our knowledge, none of them simultaneously detected the same components as ours in clinical settings. Tuffal and Michael et al adopted the LC-MS/MS method and successfully separated four thiol metabolites in healthy human plasma[21–22]. However, the 2-Oxo-CLP was not detected in their studies. Lyngby et al identified clopidogrel and its metabolites, including 2-Oxo-CLP and CAM in feline plasma. However, the study was limited to animal experiment[23]. In addition, Heestermans and Karaźniewicz-Łada et al dissected and quantified the plasma concentrations of clopidogrel, the inactive carboxyl metabolite and CAM in CAD patients[3,20]. Nevertheless, the inactive carboxyl metabolite was of limited clinical significance[24]. By comparison, the clopidogrel, 2-Oxo-CLP, and CAM are 3 essential components that represent the metabolic status of clopidogrel in clinical settings[25], thus, they should be adopted when investigating the metabolic status of clopidogrel to guide the anti-platelet treatment.
Compared with some previous methods which were built up on healthy volunteers[14,16,21–22], our strategy has been validated in 3 CAD patients who underwent stent implantation. It should be addressed that patients' condition may be complicated by accompanied with other diseases and treated with various medications, therefore, more interfering factors may occur during the determination of clopidogrel and its metabolites. Under such condition, our method is more clinically applicable, which can be directly adopted to determine the per se metabolic status of clopidogrel in patients with CAD.
Some important points should be noted: 1) 2-Oxo-CLP was found to be unstable in plasma. Considering that DTT is a well-known anti-oxidative reagent due to its reducibility of S-S bonds and addition of DTT can stabilize drugs and their metabolites[26], we used DTT to stabilize 2-Oxo-CLP immediately after collecting the blood samples. 2) 2-Oxo-CLP is subsequently hydrolyzed to form the CAM, which is also unstable and difficult to be quantitated due to its reactive thiol group[20]. Fortunately, the MPB can bind with this group and forms a stable compound of CAMD[23], allowing us to detect the concentration of CAM indirectly.
Our study has potential limitations. First, due to limited funding and very expensive standards, the selectivity was only tested once in the blank plasma in this study. However, we performed and built up this detection referring to the previous work, in which the selectivity had been performed using six different sources of blank plasma[18]. In our system, we re-checked the selectivity and found the tR values of clopidogrel and IS were similar to those in the previous study[18]. In addition, the tR values were well consistent within the patient's plasma. Thus, we believe that our system is practical to reveal the per se changes of the three analysts. Second, the difference of recovery between the lowest and the highest percent extraction was over 15%, and most recoveries were above 100%. This might be caused by the interference of sample matrix, matrix effect of human plasma, impurity of the standards, etc. However, we have to report what we had reached in the experiment, as it was limited to obtain other batches of these standards to repeat the experiments due to limited funding and expensive standards.
To summarize, our study provides an applicable method to simultaneously determine the plasma clopidogrel, 2-Oxo-CLP, and the clopidogrel active thiol metabolite, which will simplify the future pharmacokinetic study of clopidogrel, and help to fulfil the pharmacokinetic parameters-guided anti-platelet treatment.
This work was supported by National Natural Science Funding of China (Grant No. 81170181), the Jiangsu Province's Key Provincial Talents Program (Grant No. ZDRCA2016013), the Second Level of 333 High Level Talent Training Project in Jiangsu Province (Grant No. BRA2019099), Special Fund for Key R&D Plans (Social Development) of Jiangsu Province (Grant No. BE2019754), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutes.
| [1] |
Savi P, Herbert JM. Clopidogrel and ticlopidine: P2Y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis[J]. Semin Thromb Hemost, 2005, 31(2): 174–183. doi: 10.1055/s-2005-869523
|
| [2] |
Gurbel PA, Bliden KP, Hiatt BL, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity[J]. Circulation, 2003, 107(23): 2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83
|
| [3] |
Heestermans AACM, van Werkum JW, Schömig E, et al. Clopidogrel resistance caused by a failure to metabolize clopidogrel into its metabolites[J]. J Thromb Haemost, 2006, 4(5): 1143–1145. doi: 10.1111/j.1538-7836.2006.01891.x
|
| [4] |
Tang M, Mukundan M, Yang J, et al. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol[J]. J Pharmacol Exp Ther, 2006, 319(3): 1467–1476. doi: 10.1124/jpet.106.110577
|
| [5] |
Kubica A, Kozinski M, Grzesk G, et al. Genetic determinants of platelet response to clopidogrel[J]. J Thromb Thrombolysis, 2011, 32(4): 459–466. doi: 10.1007/s11239-011-0611-8
|
| [6] |
Kazui M, Nishiya Y, Ishizuka T, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite[J]. Drug Metab Dispos, 2010, 38(1): 92–99. doi: 10.1124/dmd.109.029132
|
| [7] |
Bobescu E, Covaciu A, Rus H, et al. Low response to clopidogrel in coronary artery disease[J]. Am J Ther, 2020, 27(2): e133–e141. doi: 10.1097/MJT.0000000000001099
|
| [8] |
Ying L, Wang J, Li J, et al. Intensified antiplatelet therapy in patients after percutaneous coronary intervention with high on-treatment platelet reactivity: the OPTImal Management of Antithrombotic Agents (OPTIMA)-2 Trial[J]. Br J Haematol, 2022, 196(2): 424–432. doi: 10.1111/bjh.17847
|
| [9] |
Zou X, Deng X, Wang Y, et al. Genetic polymorphisms of high platelet reactivity in Chinese patients with coronary heart disease under clopidogrel therapy[J]. Int J Clin Pharm, 2020, 42(1): 158–166. doi: 10.1007/s11096-019-00953-w
|
| [10] |
Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis[J]. JAMA, 2010, 304(16): 1821–1830. doi: 10.1001/jama.2010.1543
|
| [11] |
Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial[J]. JAMA, 2011, 305(11): 1097–1105. doi: 10.1001/jama.2011.290
|
| [12] |
Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS[J]. Eur J Cardiothorac Surg, 2018, 53(1): 34–78. doi: 10.1093/ejcts/ezx334
|
| [13] |
Liang Y, Johnston M, Hirsh J, et al. Relation between clopidogrel active metabolite levels and different platelet aggregation methods in patients receiving clopidogrel and aspirin[J]. J Thromb Thrombolysis, 2012, 34(4): 429–436. doi: 10.1007/s11239-012-0762-2
|
| [14] |
Peer CJ, Spencer SD, VanDenBerg DAH, et al. A sensitive and rapid ultra HPLC–MS/MS method for the simultaneous detection of clopidogrel and its derivatized active thiol metabolite in human plasma[J]. J Chromatogr B, 2012, 880: 132–139. doi: 10.1016/j.jchromb.2011.11.029
|
| [15] |
Karaźniewicz-Łada M, Danielak D, Teżyk A, et al. HPLC-MS/MS method for the simultaneous determination of clopidogrel, its carboxylic acid metabolite and derivatized isomers of thiol metabolite in clinical samples[J]. J Chromatogr B, 2012, 911: 105–112. doi: 10.1016/j.jchromb.2012.11.005
|
| [16] |
Silvestro L, Gheorghe M, Iordachescu A, et al. Development and validation of an HPLC-MS/MS method to quantify clopidogrel acyl glucuronide, clopidogrel acid metabolite, and clopidogrel in plasma samples avoiding analyte back-conversion[J]. Anal Bioanal Chem, 2011, 401(3): 1023–1034. doi: 10.1007/s00216-011-5147-4
|
| [17] |
Takahashi M, Pang H, Kawabata K, et al. Quantitative determination of clopidogrel active metabolite in human plasma by LC-MS/MS[J]. J Pharm Biomed Anal, 2008, 48(4): 1219–1224. doi: 10.1016/j.jpba.2008.08.020
|
| [18] |
Li Y, Song M, Hang T. Development of an LC−MS/MS method for determination of 2-oxo-clopidogrel in human plasma[J]. J Pharm Anal, 2015, 5(1): 12–17. doi: 10.1016/j.jpha.2014.07.004
|
| [19] |
Zhu Y, Zhou J. In vitro biotransformation studies of 2-oxo-clopidogrel: multiple thiolactone ring-opening pathways further attenuate prodrug activation[J]. Chem Res Toxicol, 2013, 26(1): 179–190. doi: 10.1021/tx300460k
|
| [20] |
Karaźniewicz-Łada M, Danielak D, Burchardt P, et al. Clinical pharmacokinetics of clopidogrel and its metabolites in patients with cardiovascular diseases[J]. Clin Pharmacokinet, 2014, 53(2): 155–164. doi: 10.1007/s40262-013-0105-2
|
| [21] |
Tuffal G, Roy S, Lavisse M, et al. An improved method for specific and quantitative determination of the clopidogrel active metabolite isomers in human plasma[J]. Thromb Haemost, 2011, 105(4): 696–705. doi: 10.1160/TH10-09-0582
|
| [22] |
Furlong MT, Savant I, Yuan M, et al. A validated HPLC-MS/MS assay for quantifying unstable pharmacologically active metabolites of clopidogrel in human plasma: application to a clinical pharmacokinetic study[J]. J Chromatogr B, 2013, 926: 36–41. doi: 10.1016/j.jchromb.2013.02.031
|
| [23] |
Lyngby JG, Court MH, Lee PM. Validation of a method for quantitation of the clopidogrel active metabolite, clopidogrel, clopidogrel carboxylic acid, and 2-oxo-clopidogrel in feline plasma[J]. J Vet Cardiol, 2017, 19(4): 384–395. doi: 10.1016/j.jvc.2017.03.004
|
| [24] |
Coukell AJ, Markham A. Clopidogrel[J]. Drugs, 1997, 54(5): 745–750. doi: 10.2165/00003495-199754050-00006
|
| [25] |
Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway[J]. Pharmacogenet Genomics, 2010, 20(7): 463–465. doi: 10.1097/FPC.0b013e3283385420
|
| [26] |
Tang W, Harris LC, Outhavong V, et al. Antioxidants enhance in vitro plant regeneration by inhibiting the accumulation of peroxidase in Virginia pine (Pinus virginiana Mill. )[J]. Plant Cell Rep, 2004, 22(12): 871–877. doi: 10.1007/s00299-004-0781-3
|
| [1] | Anastasia V. Poznyak, Alexey Aleksandrovich Yakovlev, Mikhail А. Popov, Alexander D. Zhuravlev, Vasily N. Sukhorukov, Alexander N. Orekhov. Coronary atherosclerotic plaque regression strategies[J]. The Journal of Biomedical Research. DOI: 10.7555/JBR.37.20230223 |
| [2] | Yao Daokuo, Gao Xiangyu, Zhao Huiqiang, Chen Hui, Wang Lexin. Multivessel coronary artery ectasia and severe calcification in a patient with pheochromocytoma: a case report[J]. The Journal of Biomedical Research, 2019, 33(1): 69-72. DOI: 10.7555/JBR.32.20170047 |
| [3] | Ling Yan, Shuang Ding, Bing Gu, Ping Ma. Clinical application of simultaneous detection of cystatin C, cathepsin S, and IL-1 in classification of coronary artery disease[J]. The Journal of Biomedical Research, 2017, 31(4): 315-320. DOI: 10.7555/JBR.31.20150152 |
| [4] | Jiawei Liao, Wei Huang, George Liu. Animal models of coronary heart disease[J]. The Journal of Biomedical Research, 2017, 31(1): 3-10. DOI: 10.7555/JBR.30.20150051 |
| [5] | Bernardo L Trigatti, Mark Fuller. HDL signaling and protection against coronary artery atherosclerosis in mice[J]. The Journal of Biomedical Research, 2016, 30(2): 94-100. DOI: 10.7555/JBR.30.20150079 |
| [6] | Rajiv Shrestha, Jing Xu, Dujiang Xie, Zhizhong Liu, Tian Xu, Fei Ye, Shiqing Din, Xuesong Qian, Song Yang, Yueqiang Liu, Feng Li, Aiping Zhang, Shaoliang Chen. Comparison of clinical outcomes of Chinese men and women after coronary stenting for coronary artery disease: a multi-center retrospective analysis of 4,334 patients[J]. The Journal of Biomedical Research, 2014, 28(5): 368-375. DOI: 10.7555/JBR.28.20120127 |
| [7] | Xuezhong Wang, Xiaoxuan Gong, Tiantian Zhu, Qiu Zhang, Yangyang Zhang, Xiaowei Wang, Zhijian Yang, Chunjian Li. Clopidogrel improves aspirin response after off-pump coronary artery bypass surgery[J]. The Journal of Biomedical Research, 2014, 28(2): 108-113. DOI: 10.7555/JBR.28.20120139 |
| [8] | Bailing Hsu. PET tracers and techniques for measuring myocardial blood flow in patients with coronary artery disease[J]. The Journal of Biomedical Research, 2013, 27(6): 452-459. DOI: 10.7555/JBR.27.20130136 |
| [9] | Min Zhang, Yan Zhang, Shuaishuai Zhu, Xiaoyu Li, Qing Yang, Hui Bai, Qi Chen. Genetic variants of the class A scavenger receptor gene are associated with coronary artery disease in Chinese[J]. The Journal of Biomedical Research, 2012, 26(6): 418-424. DOI: 10.7555/JBR.26.20110116 |
| [10] | Yangyang Zhang, Yanhu Wu, Biao Yuan, Xiang Liu, Sheng Zhao, Zhi Li, Yu Xia. Coronary artery bypass grafting with concomitant resection for carcinoma of lung[J]. The Journal of Biomedical Research, 2010, 24(1): 77-80. |
| 1. | Li P, Cao M, Liu L, et al. Analysis of the effect of CYP2C19 gene properties on the anti-platelet aggregation of clopidogrel after carotid artery stenting under network pharmacology. BMC Pharmacol Toxicol, 2024, 25(1): 34. DOI:10.1186/s40360-024-00750-w |
| Analytes | Precursor ion (m/z) | Fragment ion (m/z) | Cone voltage (V) | Collision energy (eV) |
| Clopidogrel | 322.0 | 212.0 | 22 | 15 |
| 2-Oxo-CLP | 338.0 | 183.0 | 24 | 17 |
| CAMD | 504.0 | 354.0 | 27 | 19 |
| IS | 430.0 | 372.0 | 28 | 21 |
| 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative; IS: internal standard. | ||||
| Analytes | Concentration (ng/mL) | Recovery | Matrix effect | |||
| (%) | RSD (%) | (%) | RSD(%) | |||
| Clopidogrel | 0.1 | 101.9±8.3 | 8.1 | 76.7±2.3 | 3.0 | |
| 40 | 81.5±12.0 | 14.7 | 75.8±10.0 | 13.2 | ||
| 2-Oxo-CLP | 1 | 70.2±22.1 | 31.5 | 74.6±9.7 | 13.0 | |
| 40 | 62.4±6.1 | 9.7 | 60.3±0.5 | 0.9 | ||
| CAMD | 1 | 72.2±26.9 | 37.2 | 75.1±9.7 | 12.9 | |
| 80 | 82.4±15.2 | 18.4 | 69.8±2.2 | 3.1 | ||
| All the data are presented as mean±SD. 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative; DTT: 1, 4-Dithio-DL-threitol; RSD: relative standard deviation. | ||||||
| Concentration (ng/mL) | 0.01 mol/L HCl | 0.05 mol/L HCl | 0.1 mol/L HCl | ||||||
| Extraction efficiency (%) | Matrix effect (%) | Extraction efficiency (%) | Matrix effect (%) | Extraction efficiency (%) | Matrix effect (%) | ||||
| Clopidogrel | 0.1 | 170.9±8.9 | 364.0±10.2 | 107.4±6.2 | 75.0±2.8 | 194.4±9.8 | 52.9±2.9 | ||
| 20 | 147.5±6.1 | 107.4±4.5 | 116.5±2.2 | 94.1±10.0 | 155.7±7.4 | 52.1±4.0 | |||
| 40 | 138.8±5.4 | 88.2±4.3 | 118.7±8.7 | 71.9±5.9 | 134.0±6.1 | 49.0±3.1 | |||
| 2-Oxo-CLP | 1 | 96.5±6.4 | 72.1±4.3 | 92.5±3.9 | 63.7±3.4 | 115.1±9.5 | 40.5±3.6 | ||
| 20 | 103.2±3.4 | 61.3±4.0 | 117.0±3.5 | 71.6±5.0 | 148.3±6.5 | 38.2±3.1 | |||
| 40 | 103.9±3.2 | 64.5±4.3 | 110.9±7.8 | 63.7±4.6 | 133.7±3.5 | 39.5±2.0 | |||
| CAMD | 1 | 32.1±2.3 | 84.0±2.5 | 96.7±3.2 | 70.8 ±5.3 | 93.4±13.3 | 52.2±6.3 | ||
| 40 | 34.8±1.6 | 80.7±3.8 | 123.4±3.9 | 53.0±5.8 | 171.6±5.3 | 39.7±2.1 | |||
| 80 | 34.0±2.6 | 88.4±2.3 | 120.2±7.0 | 59.3±5.0 | 167.9±5.1 | 53.8±4.6 | |||
| All the data were presented as mean±SD. 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative. | |||||||||
| Compound (ng/mL) | Calibration curve equations | Correlation coefficient (r) |
| Clopidogrel (0.05–50.0) | Pclopidogrel/PIS=0.08147×Cclopidogrel +0.001820 | 0.9984 |
| 2-Oxo-CLP (0.5–50.0) | P2-OXO-CLP/PIS=0.009094×C2-Oxo-CLP +0.0003292 | 0.9974 |
| CAMD (0.5–100.0) | PCAMD/PIS=0.01072×CCAMD+0.0004895 | 0.9979 |
| 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative. | ||
| Analyte | Nominal concentration (ng/mL) | Intra-day (n=6) | Inter-day (n=3) | |||||
| Mean assayed value (ng/mL) | Accuracy (RE [%]) | Precision (RSD [%]) | Mean assayed value (ng/mL) | Accuracy (RE [%]) | Precision (RSD [%]) | |||
| Clopidogrel | 0.1 | 0.1 | 15.2 | 4.8 | 0.1 | 12.2 | 18.7 | |
| 20 | 20.7 | 3.6 | 2.2 | 20.4 | 2.0 | 3.7 | ||
| 40 | 41.8 | 4.5 | 2.7 | 42.4 | 5.9 | 5.6 | ||
| 2-Oxo-CLP | 1 | 1.1 | 5.8 | 4.7 | 1.0 | 2.8 | 19.2 | |
| 20 | 21.2 | 5.8 | 4.0 | 20.6 | 2.9 | 6.4 | ||
| 40 | 41.0 | 2.7 | 5.8 | 42.4 | 6.0 | 7.7 | ||
| CAMD | 1 | 1.0 | 2.8 | 8.5 | 1.0 | 4.0 | 14.8 | |
| 40 | 42.0 | 5.0 | 4.3 | 42.0 | 5.1 | 8.8 | ||
| 80 | 83.0 | 3.7 | 7.2 | 84.6 | 5.8 | 5.9 | ||
| 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative; RE: relative error, RE=(nominal sample concentration–tested sample concentration)/nominal sample concentration×100%; RSD: relative standard deviation, RSD=SD value/mean value×100%. | ||||||||
| Analytes | Added (ng/mL) | Found (ng/mL) | ||||
| At –80 °C for 45 days | After three-thaw cycles | Plasma at room temperature for 8 hours | Extract at room temperature for 8 hours | Extract in the auto-sampler at 4 °C for 24 hours | ||
| Clopidogrel | 0.2 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 |
| 10 | 10.1±0.2 | 10.1±0.8 | 10.4±0.8 | 10.0±1.4 | 10.0±0.8 | |
| 2-Oxo-CLP | 2 | 2.0±0.1 | 1.9±0.2 | 2.2±0.4 | 2.1±0.2 | 2.2±0.2 |
| 50 | 50.6±1.2 | 51.0 ± 3.9 | 52.2±5.1 | 50.1±7.6 | 49.0±3.7 | |
| CAMD | 2 | 2.0±0.1 | 1.9 ± 0.2 | 2.2±0.4 | 2.0±0.2 | 2.1±0.2 |
| 50 | 50.4±1.2 | 51.5±3.8 | 51.1±4.9 | 49.6±7.7 | 50.1±3.9 | |
| All the data are presented as mean±SD. 2-Oxo-CLP: 2-oxo-clopidogrel; CAMD: clopidogrel active metabolite derivative. | ||||||