| Citation: | Zhong Caiyun, Qiu Hong, Chen Jun, Liu Hong. Effects of volatile anesthetic preconditioning on expression of NFκB-regulated genes in aged rat myocardium[J]. The Journal of Biomedical Research, 2019, 33(4): 264-270. DOI: 10.7555/JBR.32.20170071 |
Heart disease is the leading cause of death with advancing age as a predisposing risk factor. It has been reported that a 171% increase in the number of deaths caused by cardiovascular diseases in patients between 65 to 85 years of age[1]. Aging is a heterogeneous process and affects cardiomyocytes at multiple subcellular levels, including DNA damage, changes in the gene/protein expression and increased oxidative stress[2–5]. Aging has been shown to decrease myocardial tolerance to specific components of ischemia/reperfusion (I/R) injury, including oxidative stress[6– 8]. Volatile anesthetic preconditioning (APC) has been shown to protect the myocardium from I/R injury in a fashion similar to the that of ischemic preconditioning (IPC)[9– 10]. Many signaling pathways are activated in response to myocardial I/R injury. Nuclear factor (NF) κB is a pivotal transcription factor which plays a key role in oxidative stress and inflammatory response. It is activated during I/R and has been shown to play an important role in anesthetic preconditioning[10– 13]. NFκB is composed of p50 and p65 heterodimers. Under normal physiologic conditions, it is maintained in an inactive form in the cytoplasm by its inhibitors, IκB-α and other members of the IκB family. When IκB kinase is activated, IκB is phosphorylated, leading to a dissociation of NFκB and IκB, as well as ubiquitination of κB and consequent degradation of IκB by proteasomes. IκB kinase can be activated by reoxygenation, reactive oxygen species (ROS), and ischemia. When dissociated, NFκB can translocate to the nucleus and bind to consensus sites in promotor or enhancer regions of target genes to initiate transcription[11–13]. The goal of this study was to investigate whether decreased myocardial protection against I/R injury in aged heart was due to inability of activating NFκB regulated apoptotic genes during the preconditioning period.
The protocol of this study was approved by the Animal Care Committee of the University of California, Davis, and all experiments were carried out in accordance with guidelines of animal care from the National Institutes of Health.
Hearts were obtained from male Fisher rats (weight, 350–400 g, NIA). Anesthesia was induced with an intraperitoneal (IP) injection of sodium thiopental (50–75 mg/kg) and 1 000 units of heparin were used IP for anticoagulation. Because sodium thiopental has been shown not to influence preconditioning, it was chosen for initial anesthesia[14–15]. Heart was removed from experiment animal and placed in an ice-cold solution of Krebs-Henseleit buffer. The heart was then cannulated and Langendorff-perfused with Krebs-Henseleit buffer (127 mmol/L NaCl, 4.7 mmol/L KCl, 1.25 mmol/L MgCl2, 2.5 mmol/L CaCl2, 25 mmol/L NaHCO3, 10 mmol/L glucose) at a perfusion pressure of (140±10) cm H2O at (37±0.5) °C. The perfusion solution was continuously oxygenated with 95% O2–5% CO2. After cannulation, the heart was paced by placing pacing wires in the right atrium.
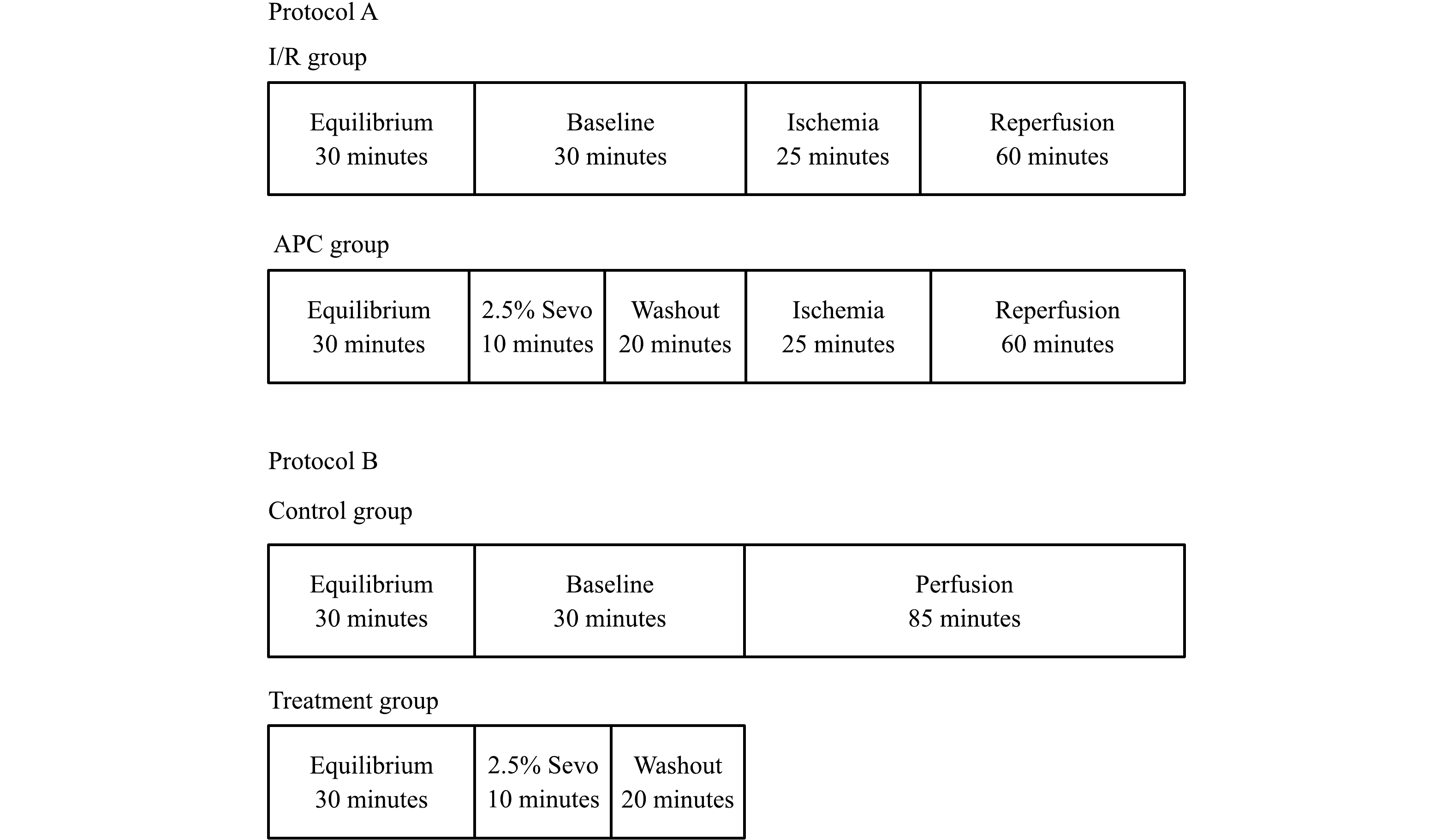
A total of 19 rats were randomly assigned to each of the three groups: (1) the control group: 130 minutes of continuous perfusion; (2) the I/R group: 30 minutes of equilibration, 30 minutes of baseline, 25 minutes of global ischemia, 60 minutes of reperfusion; and (3) the APC group: the same as the I/R group except 10 minutes of sevoflurane exposure followed by a 20 minutes washout was included immediately before 25 minutes of global ischemia (Fig. 1). Treatment group: the same as APC group except there was no I/R and the myocardial tissues were obtained at the end of washout. 2.5% sevoflurane was delivered to the gas mixture via a standard Sevotec 5 vaporizer (DatexOhmeda, Milwaukee, WI) with a final concentration of 0.40±02 mmol/L. Global ischemia was induced by stopping all flow to the heart. Atria was paced at 5 Hz during all phases of the experiment except global ischemia. Episodes of ventricular fibrillation were mechanically converted when they occurred.
A latex balloon filled with water and connected to a pressure transducer (Medex, Dublin, CA, USA) was used to measure left ventricular pressures. It was inserted into the left ventricle via the left atrial appendage through the mitral valve. The balloon volume was adjusted to produce a left ventricular enddiastolic pressure (LVEDP) of 5–7 mmHg during the equilibration period. All the pressures were recorded using Powerlab 4/20 hardware with an amplifier (AD Instruments, Colorado Springs, CO, USA) and Chart for Windows version 4.0.4 software (AD Instruments). Left ventricular developed pressure (LVDP) was used as the indication of left ventricular systolic function.
At the end of study, the LV muscles of the I/R group and APC group were cut into 2 mm thickness of sections. The sections were immersed in 1% 2,3,5-triphenyltetrazolium chloride staining solution and incubated at 37 °C for 20 minutes. Normal noninfarcted myocardium stains a bright red. This is caused by reduction of 2,3,5-triphenyltetrazolium chloride by dehydrogenases present in viable tissue[10]. The myocardial sections were scanned into a computer using Adobe Photoshop software (Adobe, San Jose, CA, USA). Standard computer planometric analysis and the NIH image 1.62 (public domain) were used to determine infarct areas. Infarct size was determined dividing the necrotic area by the total slice area of LV.
The levels of IκB-α, ICAM-1, and iNOS in the hearts were measured by using Western blot analyses. Heart cytosolic proteins (IκB-α and iNOS) and microsomal protein (ICAM-1) were loaded and separated on 7.5%–10% SDS-PAGE, followed by transblotting to an ImmunBlot PVDF membrane (Bio-Rad, Hercules, CA, USA). The membrane was subsequently probed with primary IκB-α, ICAM-1, and iNOS antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1 : 1 000. Horseradish peroxidase–conjugated secondary antibody was added at 1 : 3 000 dilution. An enhanced chemiluminescence detection kit (AmerSham Pharmacia Biotech, Inc., Piscataway, NJ, USA) was used to develop the blots. The immunoreactive protein bands were quantified by densitometry after exposure on autoradiography film.
Statistical analysis of variance for repeated measures was used to test the differences between treatments. When differences between the two treatments were found, the unpaired t test was used to determine the times at which differences between treatments occurred. The t test was used only across different treatments for a particular time. Under these circumstances, the t test and multiple-comparison tests provide identical results for two treatments. Results were reported as mean±SD. For all comparisons, P<0.05 were considered statistically significant.
The LVDP represents the LV systolic function. The average LVDP was measured during 60 minutes of reperfusion during the preischemic period. The LVDP was significantly decreased during of reperfusion to (25±6) mmHg (P<0.05) at the end of 60 minutes reperfusion in the I/R group. The LVDP was significantly decreased during reperfusion to (22±7) mmHg (P<0.05) at the end of 60 minutes of reperfusion in the treatment group. There was no significant difference between control and APC hearts in the aged hearts [(25±6) mmHg vs. (22±7) mmHg, P>0.05, Fig. 2)]. The LVEDP represents the LV diastolic function and was significantly elevated during reperfusion and reached (97±12) mmHg in I/R group and (93±7) mmHg in APC group with no statistical differences between the two groups (Fig. 3). The infarct size was (27±3)% in I/R group and was (26±4)% in APC group, there were no statistical differences (P>0.05).
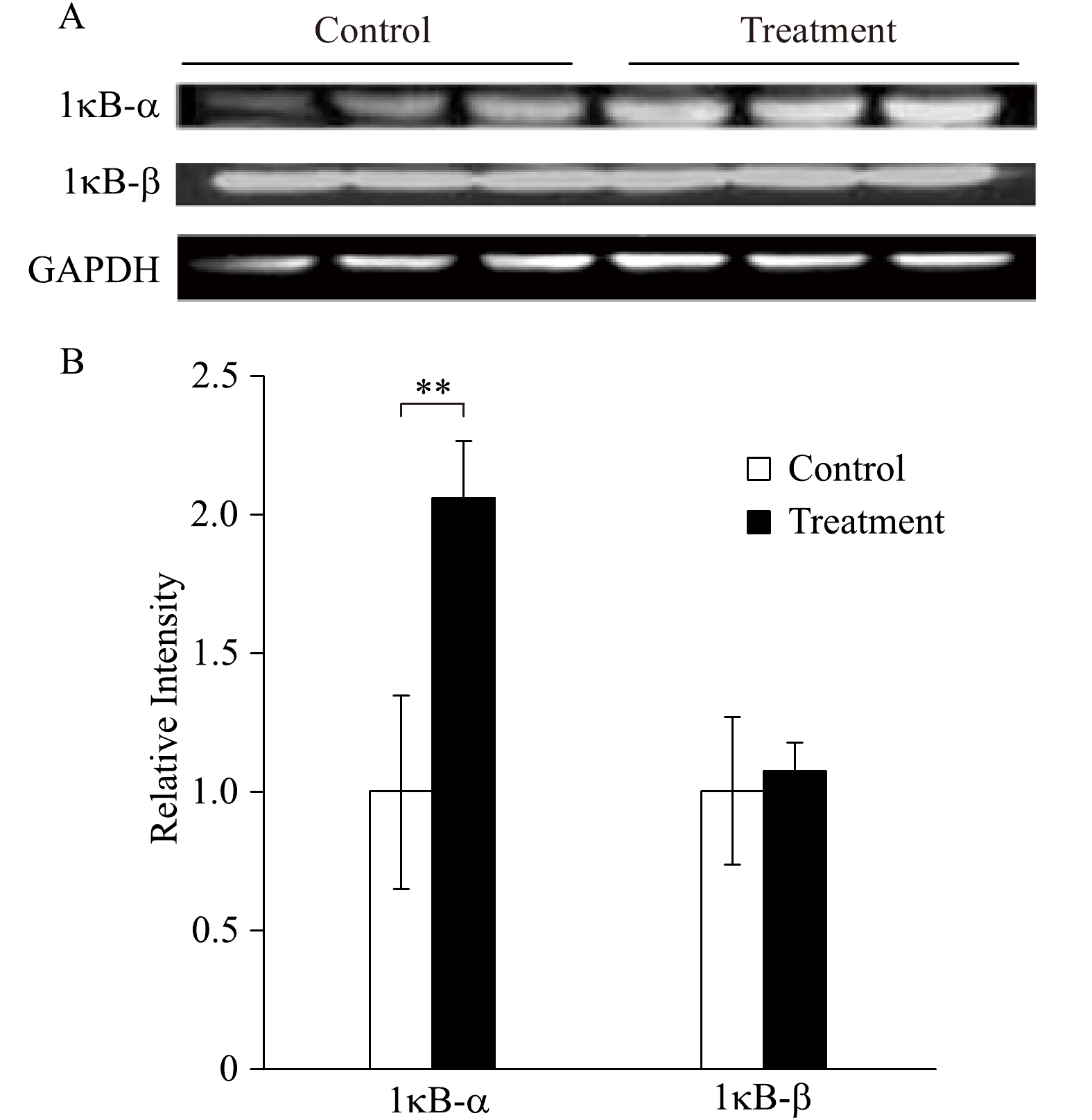
Fig. 4 represents the levels of IκB in the cytosol of aging rats. After anesthetic preconditioning with 2.5% sevoflurane, the level of IκB-α was elevated compared to the control group that no sevoflurane was exposed, while there were no differences in the level of IκB-β between the control and treatment group (Fig. 4).
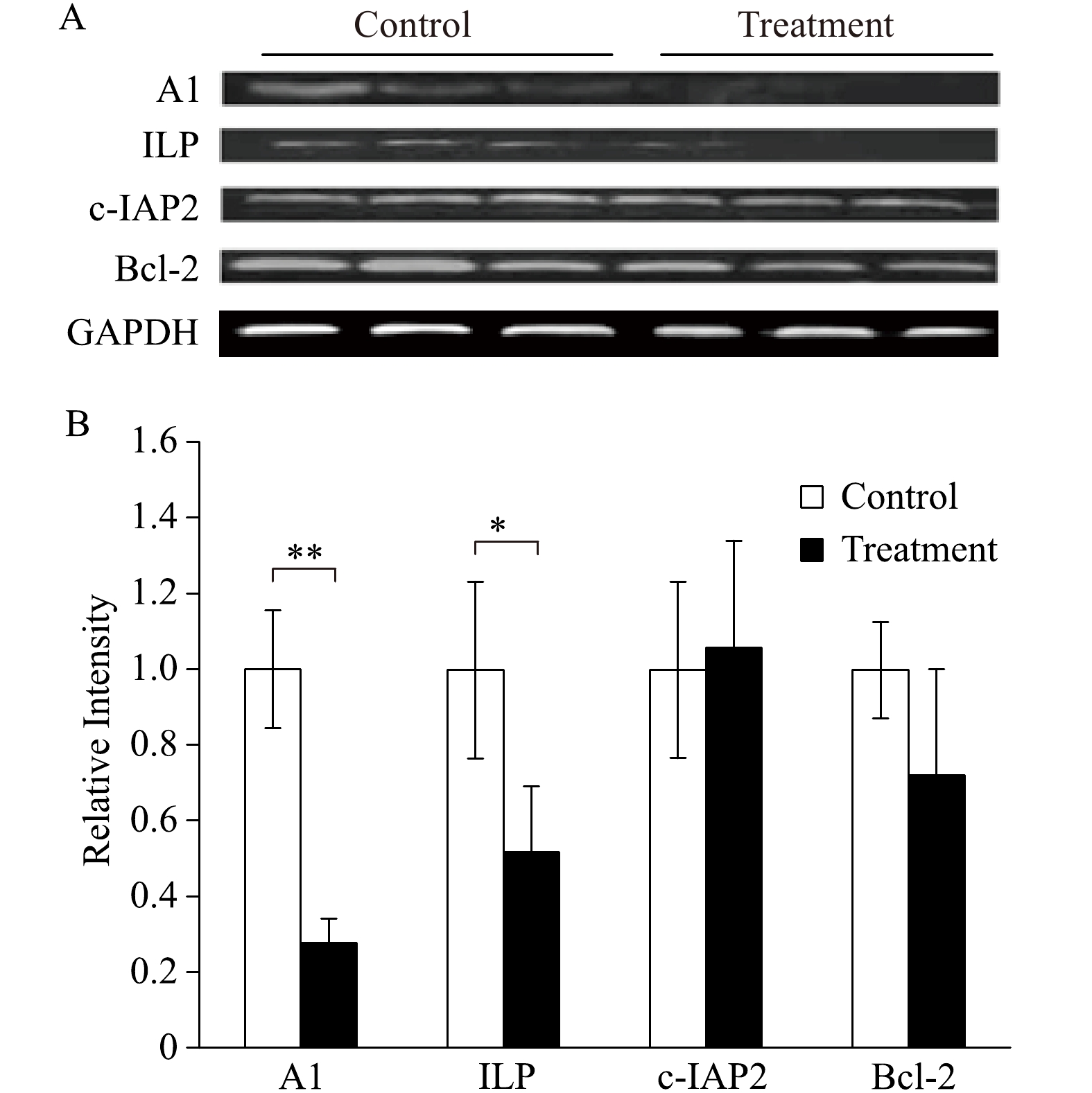
There were significant decreases of expression of A1 and ILP, while there were no differences in the expression of c-IAP2 and Bcl-2 (Fig. 5). The decreases in anti-apoptotic gene expressions are consistent with the increased levels of IκB-α and decreased myocardial protection.
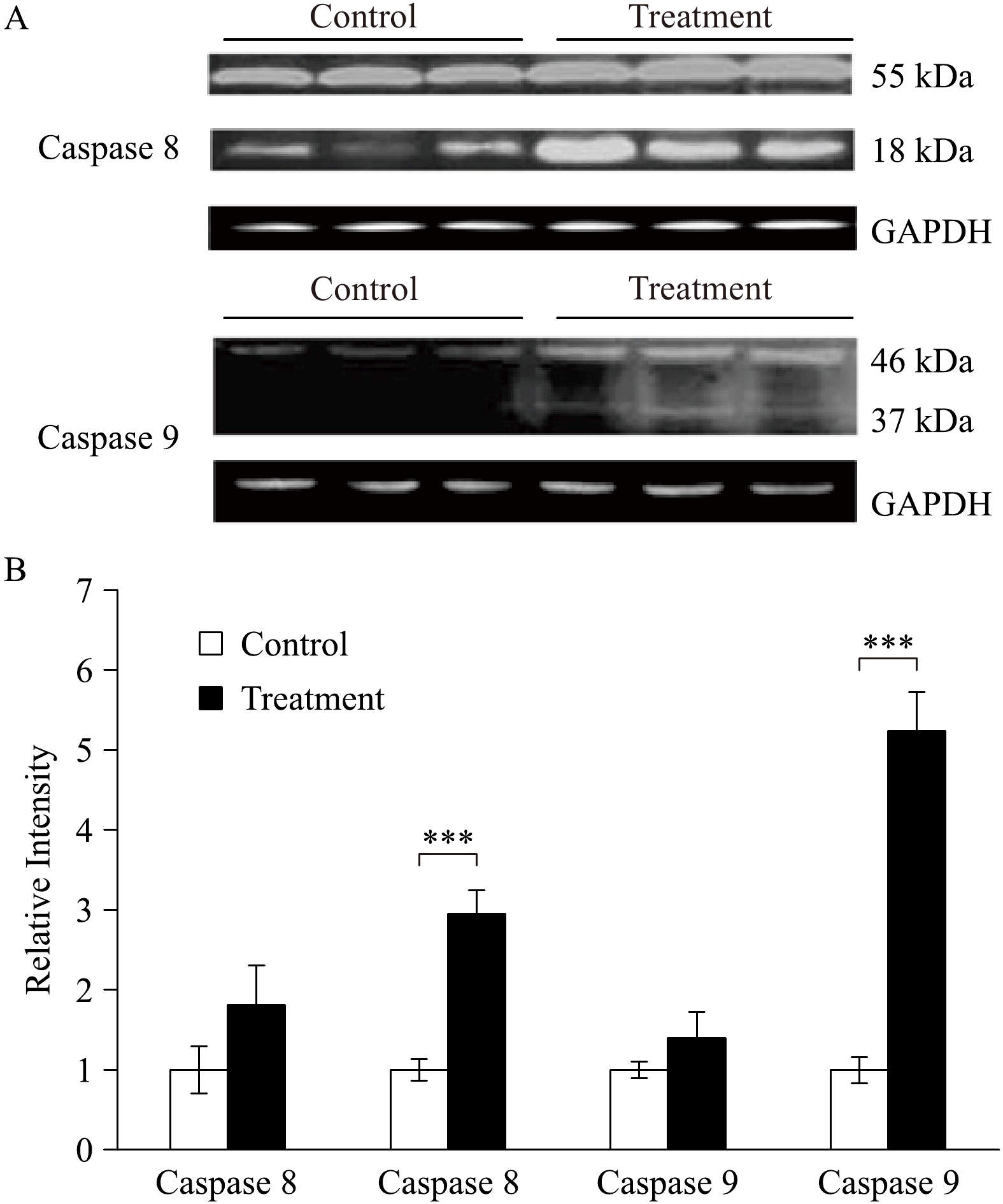
Caspases represent the apoptosis. In this study, caspase-8 (18 kDa) and caspase 9 (37 kDa) were significantly increased in the group treated with 2.5% sevoflurane compared to the control group that received no treatment (Fig. 6).
Our previous results and current results have demonstrated that volatile anesthetic preconditioning failed to protect aged myocardium from I/R injury[16]. We investigated the roles of NFκB and its regulated factors on myocardial protection in aged population. Our results are consistent with others[17] and our hypothesis that the inability to activate NFκB regulated apoptotic genes during preconditioning period is the key to the failed myocardial protection against I/R injury.
The percentage of the world's elderly population is increasing. As a result, the number of elderly patients undergoing surgical procedures is increasing[18]. This has important implications for intraoperative cardioprotection because there are metabolic and structural differences in the senescent myocardium[19]. Aging is a major cause of congestive heart failure. More than 75% of patients with congestive heart failure are over 65 years of age, and the elderly contribute to a significant increase in cardiovascular mortality and heart failure[20].
Studies demonstrated that volatile anesthetic preconditioning protects myocardium from I/R injury. APC not only protects against I/R injury but also improves functional recovery, decrease post-ischemic myocardial stunning, and attenuate myocardial apoptosis. These protective effects were observed with sevoflurane[13– 17,21]. On the other hand, there are studies showing that APC failed to protect myocardium against I/R injury in aged population[16– 17,22]. This study is consistent with the above-mentioned studies that APC was unable to achieve myocardial protection in aged rats.
NF-кB is a pivotal inducible transcription factor that regulates the expression of many genes involved in the processes of inflammation and apoptosis. Depending on the activation time and degree of NFκB, it can be either protective or harmful. Ischemia and reperfusion result in activation of NFκB, with deleterious consequences such as induction of inflammatory cytokines and cleavage of pro-caspases[12– 13]. But NFκB activation at a lower level prior to I/R could be protective[13]. NFκB blocker attenuates the APC induced myocardial protection when given prior to preconditioning[10,23]. In another study, the authors found that there is an elevated baseline level of reactive oxygen species (ROS) and the inhalational anesthetic preconditioning was unable to further increase ROS activity in aged myocardium compared to young myocardium[17]. ROS is considered to be one of the potent activators for NF-кB.
One of the protective mechanisms of anesthetic preconditioning is its involvement in anti-apoptotic process. Results from one study demonstrated that sevoflurane stimulated upregulation of Bcl-2 protein expression independent of ischemia and reperfusion in young myocardium[13]. This upregulation of Bcl-2 in the preconditioning period was abolished by inhibition of NF-κB, providing evidence that activation of NF-κB by sevoflurane stimulated the production of this protein. This was further supported by another study that the anti-apoptosis protein Bcl-2 could be induced by NF-κB during preconditioning[24]. Grünenfelder et al has also reported that blockade of caspase-3 could ameliorate reperfusion injury by upregulating Bcl-2 and inhibiting TNF-α[25]. We can suggest that sevoflurane-induced cardioprotection is triggered by producing reduced oxidative stress which activates NF-KB during the preconditioning periods and promotes the expression of anti-apoptotic genes that inhibit caspase-8 and caspase-9, and prevents myocardial I/R injury such as apoptosis during reperfusion in young myocardium. However, in aged myocardium, we observed the opposite. In parallel with the increased expression of IKB-α and the decreased expression of Bcl-2 related anti-apoptosis protein A1 and ILP, the marker protein of apoptosis caspase-8, and caspase-9 were significantly increased by inhibition of NF-κB and downregulation of antiapoptotic gene expressions.
There are some limitations in this study. We did not try to compare aged hearts with young hearts in this study. We only used inhaled sevoflurane, and we do not know whether all the volatile anesthetics are equal in efficacy concerning APC. Although several studies have demonstrated efficacy of APC in human surgical conditions, it is unknown whether NF-κB activation occurs in human APC. In addition, these findings are applicable to early APC, not delayed APC in which I/R occurs 24 to 48 hours following anesthetic exposure. We measured IκB, the NFκB inhibitor, not NFκB.
In conclusion, the results of this study demonstrated that the volatile anesthetic preconditioning with sevoflurane fail to produce protective effect against myocardial I/R in aged rats. IκB-α, the NFκB inhibitor, was upregulated in the inhalational anesthetic group. Furthermore, our results indicated that anti-apoptotic proteins such as A1 and ILP were significantly downregulated during preconditioning period. Our data suggest that volatile anesthetic preconditioning with sevoflurane failed to protect myocardial I/R injury in aged rats, which may be due to the inhibition of NFκB and downregulation of anti- apoptotic genes.
This work was supported by the Department of Anesthesiology and Pain Medicine, University of California Davis Health.
| [1] |
Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics-2014 update: a report from the American Heart Association[J]. Circulation, 2014, 129(3): e28–e292.
|
| [2] |
Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing[J]. Cardiovasc Res, 2009, 83(2): 247–261. doi: 10.1093/cvr/cvp033
|
| [3] |
Hariharan N, Sussman MA. Cardiac aging- Getting to the stem of the problem[J]. J Mol Cell Cardiol, 2015, 83: 32–36. doi: 10.1016/j.yjmcc.2015.04.008
|
| [4] |
Liu X, Lei J, Wang K, et al. Mitochondrial Omi/HtrA2 promotes caspase activation through cleavage of HAX-1 in aging heart[J]. Rejuvenation Res, 2017, 20(3): 183–192. doi: 10.1089/rej.2016.1861
|
| [5] |
Bıçakçı H, Sarsılmaz M, Ocaklı S, et al. Investigation of the effects of aging on the expression of aquaporin 1 and aquaporin 4 protein in heart tissue[J]. Anatol J Cardiol, 2017, 17(1): 18–23.
|
| [6] |
Lesnefsky EJ, Gudz TI, Moghaddas S, et al. Aging decreases electron transport complex Ⅲ activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site[J]. J Mol Cell Cardiol, 2001, 33(1): 37–47. doi: 10.1006/jmcc.2000.1273
|
| [7] |
Villmow M, Klöckner U, Heymes C, et al. NOS1 induces NADPH oxidases and impairs contraction kinetics in aged murine ventricular myocytes[J]. Basic Res Cardiol, 2015, 110(5): 506.
|
| [8] |
Ma L, Zhu J, Gao Q, et al. Restoring pharmacologic preconditioning in the aging heart: role of mitophagy/autophagy[J]. J Gerontol A Biol Sci Med Sci, 2017, 72(4): 489–498.
|
| [9] |
Kersten JR, Schmeling TJ, Pagel PS, et al. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase[J]. Anesthesiology, 1997, 87(2): 361–370. doi: 10.1097/00000542-199708000-00024
|
| [10] |
Konia MR, Schaefer S, Liu H. NF-Kappa B inhibition provides additional protection ischemia-reperfusion injury in delayed sevoflurane preconditioning[J]. Eur J Anaesthesiol, 2009, 26(6): 496–503 P. doi: 10.1097/EJA.0b013e328324ed2e
|
| [11] |
Xuan YT, Tang XL, Banerjee S, et al. Nuclear factor-kappaB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits[J]. Circ Res, 1999, 84(9): 1095–1109. doi: 10.1161/01.RES.84.9.1095
|
| [12] |
Zhong C, Zhou Y, Liu H. Nuclear factor kappaB and anesthetic preconditioning during myocardial ischemia-reperfusion[J]. Anesthesiology, 2004, 100(3): 540–546. doi: 10.1097/00000542-200403000-00012
|
| [13] |
Wang C, Xie H, Liu X, et al. Role of NF-κB in volatile anesthetic preconditioning with sevoflurane during myocardial ischemia/reperfusion in rats[J]. Eur J Anaesthesiol, 2010, 27(8): 747–756.
|
| [14] |
Lutz M, Liu H. Inhaled sevoflurane produces better delayed myocardial protection at 48 versus 24 hours after exposure[J]. Anesth Analg, 2006, 102(4): 984–990. doi: 10.1213/01.ane.0000198568.79079.4c
|
| [15] |
Deyhimy DI, Fleming NW, Brodkin IG, et al. Anesthetic preconditioning combined with postconditioning offers no additional benefit over preconditioning or postconditioning alone[J]. Anesth Analg, 2007, 105(2): 316–324. doi: 10.1213/01.ane.0000267524.71445.e7
|
| [16] |
Sniecinski R, Liu H. Reduced efficacy of volatile anesthetic preconditioning with advanced age in isolated rat myocardium[J]. Anesthesiology, 2004, 100(3): 589–597. doi: 10.1097/00000542-200403000-00019
|
| [17] |
Nguyen LT, Rebecchi MJ, Moore LC, et al. Attenuation of isoflurane-induced preconditioning and reactive oxygen species production in the senescent rat heart[J]. Anesth Analg, 2008, 107(3): 776–782. doi: 10.1213/ane.0b013e318180419d
|
| [18] |
Winker MA. Aging in the 21st century: a call for papers[J]. Arch Neurol, 2002, 59(4): 518–519. doi: 10.1001/archneur.59.4.518
|
| [19] |
Swynghedauw B, Besse S, Assayag P, et al. Molecular and cellular biology of the senescent hypertrophied and failing heart[J]. Am J Cardiol, 1995, 76(13): 2D–7D. doi: 10.1016/S0002-9149(99)80484-6
|
| [20] |
Chang WT, Chen JS, Tsai MH, et al. Interplay of aging and hypertension in cardiac remodeling: a mathematical geometric model[J]. PLoS One, 2016, 11(12): e0168071. doi: 10.1371/journal.pone.0168071
|
| [21] |
Liu H, Wang L, Eaton M, et al. Sevoflurane preconditioning limits intracellular/mitochondrial Ca2+ in ischemic newborn myocardium[J]. Anesth Analg, 2005, 101(2): 349–355. doi: 10.1213/01.ANE.0000154197.24763.EC
|
| [22] |
Liu H, Moore PGK. KATP channel blocker does not abolish the protective effect of Na+/H+ exchange 1 inhibition against ischaemia/reperfusion in aged myocardium[J]. Eur J Anaesthesiol, 2010, 27(8): 740–746.
|
| [23] |
Xie H, Wang C, Wu X, et al. Parthenolide attenuates LPSinduced activation of NF-κB in a time-dependent manner in rat myocardium[J]. J Biomed Res, 2012, 26(1): 37–43. doi: 10.1016/S1674-8301(12)60005-0
|
| [24] |
Choi H, Kim SH, Chun YS, et al. In vivo hyperoxic preconditioning prevents myocardial infarction by expressing bcl-2[J]. Exp Biol Med (Maywood), 2006, 231(4): 463–472. doi: 10.1177/153537020623100412
|
| [25] |
Grünenfelder J, Miniati DN, Murata S, et al. Upregulation of Bcl-2 through caspase-3 inhibition ameliorates ischemia/reperfusion injury in rat cardiac allografts[J]. Circulation, 2001, 104(12 Suppl 1): I202–I206.
|
| [1] | Wang Le Yi, McKelvey George M., Wang Hong. Multi-outcome predictive modelling of anesthesia patients[J]. The Journal of Biomedical Research, 2019, 33(6): 430-434. DOI: 10.7555/JBR.33.20180088 |
| [2] | Nina Schloemerkemper. Psychotic due to bath salts and methamphetamines: emergency cesarean section under general anesthesia[J]. The Journal of Biomedical Research, 2018, 32(4): 311-313. DOI: 10.7555/JBR.32.20180023 |
| [3] | Jun Ni, Hongjian Lu, Xiao Lu, Minghui Jiang, Qingyun Peng, Caili Ren, Jie Xiang, Chengyao Mei, Jianan Li. The evolving concept of physiological ischemia training vs. ischemia preconditioning[J]. The Journal of Biomedical Research, 2015, 29(6): 445-450. DOI: 10.7555/JBR.29.20140142 |
| [4] | Guoliang Meng, Jing Wang, Yujiao Xiao, Wenli Bai, Liping Xie, Liyang Shan, Philip K Moore, Yong Ji. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats[J]. The Journal of Biomedical Research, 2015, 29(3): 203-213. DOI: 10.7555/JBR.28.20140037 |
| [5] | Lintao Wang, Yanyan Peng, Kaikai Shi, Haixiao Wang, Jianlei Lu, Yanli Li, Changyan Ma. Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis[J]. The Journal of Biomedical Research, 2015, 29(2): 132-138. DOI: 10.7555/JBR.27.20120115 |
| [6] | Pengpeng Jin, Xiaoli Wang, Fei Chang, Yinyang Bai, Yingchun Li, Rong Zhou, Ling Chen. Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats[J]. The Journal of Biomedical Research, 2013, 27(2): 135-144. DOI: 10.7555/JBR.27.20120076 |
| [7] | Xiaozheng Zhong, Xiaoyu Li, Lingling Qian, Yiming Xu, Yan Lu, Jing Zhang, Nan Li, Xudong Zhu, Jingjing Ben, Qing Yang, Qi Chen. Glycine attenuates myocardial ischemia-reperfusion injury by inhibiting myocardial apoptosis in rats[J]. The Journal of Biomedical Research, 2012, 26(5): 346-354. DOI: 10.7555/JBR.26.20110124 |
| [8] | Ashish Kumar Sharma, Arshee Munajjam, Bhawna Vaishnav, Richa Sharma, Ashok Sharma, Kunal Kishore, Akash Sharma, Divya Sharma, Rita Kumari, Ashish Tiwari, Santosh Kumar Singh, Samir Gaur, Vijay Singh Jatav, Barthu Parthi Srinivasan, Shyam Sunder Agarwal. Involvement of adenosine and standardization of aqueous extract of garlic (Allium sativum Linn.) on cardioprotective and cardiodepressant properties in ischemic preconditioning and myocardial ischemia-reperfusion induced cardiac injury[J]. The Journal of Biomedical Research, 2012, 26(1): 24-36. DOI: 10.1016/S1674-8301(12)60004-9 |
| [9] | Rufeng Xie, Lizhong Wang, Hongguang Bao. Crystalloid and colloid preload for maintaining cardiac output in elderly patients undergoing total hip replacement under spinal anesthesia[J]. The Journal of Biomedical Research, 2011, 25(3): 185-190. DOI: 10.1016/S1674-8301(11)60024-9 |
| [10] | Lingyun Li, Jing Chi, Feng Zhou, Dandan Guo, Fang Wang, Genyan Liu, Chun Zhang, Kun Yao. Human herpesvirus 6A induces apoptosis of HSB-2 cells via a mitochondrion-related caspase pathway[J]. The Journal of Biomedical Research, 2010, 24(6): 444-451. DOI: 10.1016/S1674-8301(10)60059-0 |
| 1. | Hong J, Wang L, Zheng Q, et al. The Recent Applications of Magnetic Nanoparticles in Biomedical Fields. Materials (Basel), 2024, 17(12): 2870. DOI:10.3390/ma17122870 |
| 2. | Ndraha N, Lin HY, Tsai SK, et al. The Rapid Detection of Salmonella enterica, Listeria monocytogenes, and Staphylococcus aureus via Polymerase Chain Reaction Combined with Magnetic Beads and Capillary Electrophoresis. Foods, 2023, 12(21): 3895. DOI:10.3390/foods12213895 |
| 3. | Zhou J, Gui Y, Lv X, et al. Nanomaterial-Based Fluorescent Biosensor for Food Safety Analysis. Biosensors (Basel), 2022, 12(12): 1072. DOI:10.3390/bios12121072 |