| Citation: | Ling Chen, Ting Ma, Liang Wang, Lixin Wang, Minmin Li, Rong Zhu. Unilateral pleural effusion secondary to Takayasu arteritis: a case report and literature review[J]. The Journal of Biomedical Research, 2022, 36(2): 141-144. DOI: 10.7555/JBR.36.20210190 |
Takayasu arteritis (TA) is a type of nonspecific chronic large vessel vasculitis characterized by granulomatous inflammation in the vessel wall of the aorta and its major branches[1]. The pathophysiological progression of full-thickness inflammation of the vessel wall and the subsequent fibrosis usually occurs with vascular stenosis and/or occlusion, causing ischemia of the corresponding organs, which is associated with a high mortality[2]. The involvement of pulmonary artery is not rare in patients with TA[3–4]. Patients with pulmonary artery involvement commonly present with respiratory symptoms[5] , with rare reports of pleural effusion. Herein, we report a case of unilateral pleural effusion as the main manifestation to improve clinicians' understanding of TA.
The case report was approved by the Ethics Committee of Huai'an First People's Hospital (Ethics approval No. YX-2021-056-01).
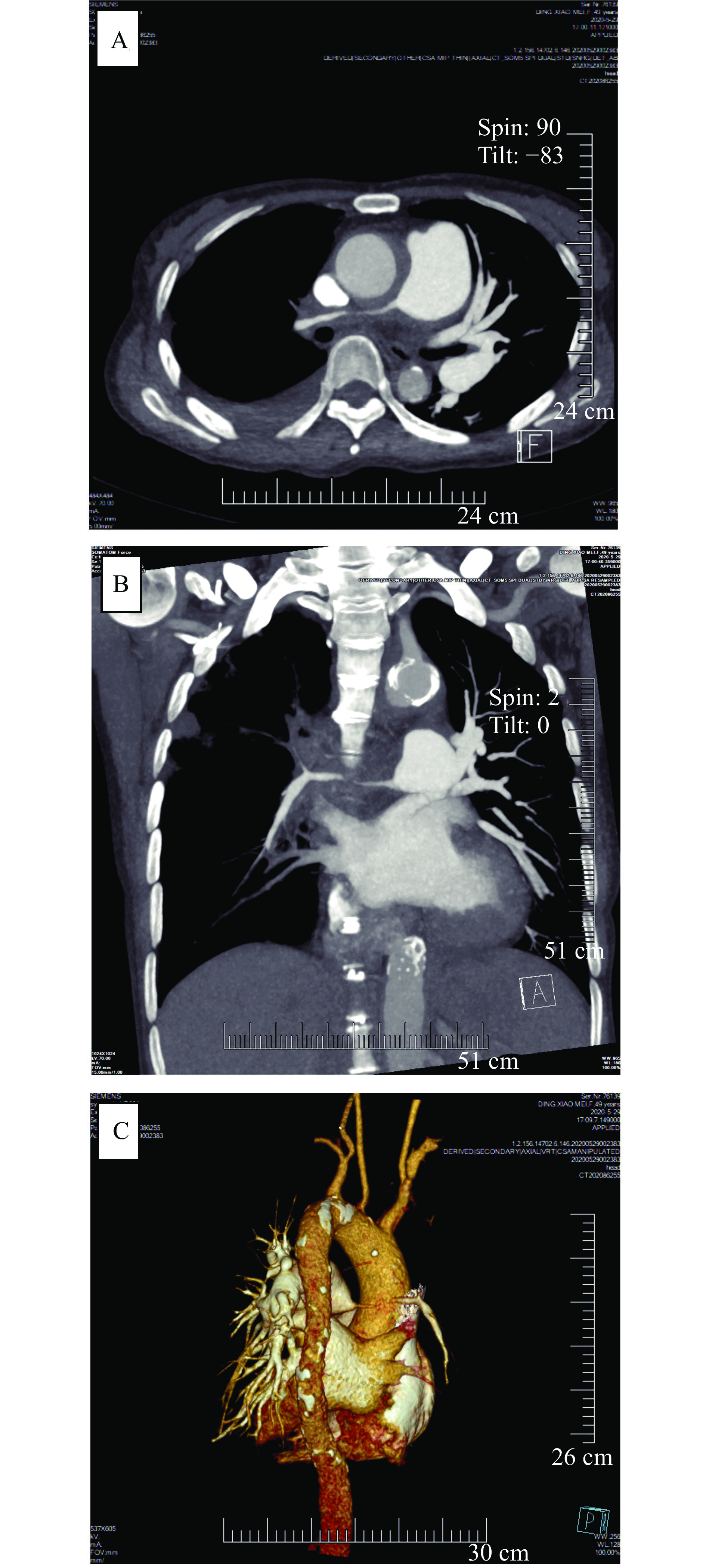
A 49-year-old female was admitted to our hospital in May 2020 due to cough and expectoration (mainly white sticky sputum) with chest pain of no obvious cause for four months. The efficacy of anti-infective therapy in local hospitals was poor. Blood routine examination before admission showed moderate anemia. Chest CT showed a large amount of pleural effusion on the right side, main pulmonary artery dilation with enlarged left hilum, and right-side pneumonia. The patient had a history of anemia for more than 20 years without receiving standardized treatment. Physical examination: T, 36.2 °C; P, 98/minute; R, 20/minute; BP, 100/55 mmHg. The respiratory movement of the right lower lung was weakened. Tremor was weakened. There was percussion dullness. Breath sound was low, and no wet and dry rales were heard.
The patient's laboratory and imaging test results are as follows. Inflammatory biomarkers: C-reactive protein, 26.2 mg/L; erythrocyte sedimentation rate, 61 mm/h;
The main manifestations of this patient were cough, expectoration, and chest pain. Echocardiography showed pulmonary hypertension. Pulmonary artery CTA and vascular ultrasound examination showed pulmonary artery and systemic vascular stenosis and occlusion, excluding congenital artery stenosis, atherosclerosis, thromboangiitis obliterans, Behcet's disease and nodular polyarteritis, and the diagnosis of TA was made. Methylprednisolone (40 mg, orally) and cyclophosphamide (0.4 g, intravenously) were administered for treatment. After being discharged from the hospital, the patient was followed up every month to receive 0.4 g cyclophosphamide intravenously and have her glucocorticoid dosage adjusted. The patient had no symptoms of cough, sputum, or chest pain, and the pleural effusion disappeared half a year later. One and a half years later, her hemoglobin level was 120 g/L.
The etiology of TA is thought to be related to genetics[6], endocrine abnormalities, immune dysfunction, and cytokine inflammation after infection (Streptococcus, Mycobacterium tuberculosis, virus, etc.)[7]. The clinical symptoms of TA are generally nonspecific and vary depending on the affected vessels[8] and the severity of the disease[4]. The diagnosis of TA mainly relies on clinical and imaging findings showing the pathological changes[9]: wall thickening, thrombus formation, stenotic and occlusive lesions due to inflammation and endothelial damage, as well as destruction of the muscularis and elastic layers caused by dilatation and aneurysms[7]. Glucocorticoids (GC), as a first line treatment, is the most effective drug for TA[9]. As experience has shown that the cumulative GC demand in TA is high, GC-sparing treatment with moderately potent immunosuppressants, such as methotrexate, azathioprine, and mycophenolate mofetil, is recommended to TA patient from the time of initial diagnosis[10].
The incidence of pulmonary arteritis (PA) in patients with TA varies greatly among studies (14% to 86%)[11]. Approximately, half of PA patients suffer from overt pulmonary hypertension (PH) during their disease course, mostly secondary to pulmonary artery stenosis or occlusion[4,12]. The CTA results of this patient showed lesions of varying degrees in the aorta, pulmonary, common carotid, and subclavian arteries, and the pulmonary artery in the middle and upper lobes of the right lung was invisible. Also, echocardiography indicated a moderate to severe pulmonary hypertension, so PA was considered.
Unlike conventional TA, PA is mainly characterized by respiratory symptoms[5]: dyspnea (68% to 83%), cough (20% to 66.7%), hemoptysis (20% to 57%), and chest pain (17% to 48%)[5,12–13]. Our patient had cough and chest pain, which is consistent with previous reports, but she also had a large unilateral pleural effusion. Using "Takayasu arteritis" and "pleural effusion" as keywords, we retrieved 5 related papers in the Wanfang, CNKI, and PubMed databases, and compiled the data of the 5 patients reported in the literature (Table 1)[14–18]. Combining the data from both literature and this case, it can be summarized that TA with pleural effusion is more frequent in women and has a fairly large age span. The patients under 45 years of age may have fever and be easily misdiagnosed as tuberculosis, while those over 45 years of age tend to have pulmonary artery involvement and present with respiratory and chest pain manifestations. The most commonly involved artery is the subclavian artery, which may be vasodilated proximally. The most common site is the left side, and the nature is exudative, ranging from small to large amounts. Unilateral pleural effusion may be related to pleural irritation due to pathological changes in the vessels anatomically proximal to the large arteries on the left side of the thorax[16–17].
| Author | Kawai et al[14] | Achari et al[15] | Schattner et al[16] | Gui et al[17] | Liang et al[18] |
| Gender | Male | Female | Female | Female | Female |
| Age (years) | 32 | 32 | 35 | 58 | 72 |
| Physical examination | Pulselessness; Vascular murmur | _ | Vascular murmur; Splenomegaly | Vascular murmur; Pleural effusion signs; Upper limb blood pressure cannot be measured. | Pleural effusion signs |
| Laboratory and imaging examinations | |||||
| Hb (g/dL) | – | 9.2 | 9.1–7.6 | 12.1 | 12.4 |
| CRP (mg/L) | 3+ | – | 102 | 42 | 8.68 |
| ESR (mm/h) | >100 | 60 | 140 | 23 | 23 |
| Autoimmunity | Negative | Negative | Anti-smooth muscle antibodies (+) | Negative | Anti-PM-Sclanmibody (±) |
| Pleural effusion | Left; Exudative; Small amount; Recurring with disease activity | Left; Exudative | Left; Small amount; Exudative | Left; Massive; Bloody | Left; Massive; Exudative |
| Involved narrowed or occluded arteries | Subclavicul bilaterally | Right subclavian; Left coronary artery | Proximal aorta; Right carotid | Subclavicul bilaterally; Right upper pulmonary | Upper and lower left pulmonary artery |
| Treatment | Misdiagnosed as tuberculosis, pleural effusion recurring along with irregular corticosteroids application, and after TA diagnosis, corticosteroids given again. | Prednisolone 60 mg daily with methotrexate 2.5 mg weekly increasing the dose to 7.5 mg weekly a month. | Prednisone 50 mg/day and methotrexate 7.5 mg/week tapered over months to 5 mg/day prednisone and aspirin. | Methylprednisolone 80 mg, 1 time/8 hours, IV, gradually reduced to prednisone 20 mg, 2 times/day orally, cyclophosphamide 0.4 g IV, with torasemide and other symptomatic supportive treatment. | Methylprednisolone 40 mg qd×7 days, intravenous drip, gradually reduced to prednisone acetate tablets 30 m/day orally and hydroxychloroquine sulfate 0.2 g bid. |
| Hb: hemoglobin; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; IV: intravenous; qd: once a day; bid: twice a day. | |||||
The production of pleural fluid comes mainly from the filtration of the wall pleura capillaries, while the absorption of pleural fluid depends on the reabsorption of the pleura wall (under normal conditions, the role of the dirty pleura in the circulation of pleural fluid is smaller)[19]. Pleural fluid production and absorption are normally in a dynamic equilibrium, and when disturbed in some pathological conditions, pleural effusion is formed. The etiology of pleural effusion varies, and in general, the first step in diagnosis is to determine whether the nature of the effusion is transudative or exudative. Exudative pleural effusions are usually prioritized as pneumonia, tuberculosis, or malignancy[20]. When looking for causes, we often focus on common causes and miss rare or uncommon causes. In this paper, we report a rare clinical presentation (unilateral pleural effusion) of a rare disease (i.e., TA), and review the relevant literature to summarize the general characteristics of such a disease, hoping to improve clinicians' knowledge and understanding of TA and pleural effusion. However, because there are few relevant reports, the etiology and specific pathogenesis cannot be clarified, and more clinical data and research support are needed.
| [1] |
Watts RA, Hatemi G, Burns JC, et al. Global epidemiology of vasculitis[J]. Nat Rev Rheumatol, 2022, 18(1): 22–34. doi: 10.1038/s41584-021-00718-8
|
| [2] |
Alnabwani D, Patel P, Kata P, et al. The epidemiology and clinical manifestations of Takayasu arteritis: a descriptive study of case reports[J]. Cureus, 2021, 13(9): e17998. doi: 10.7759/cureus.17998
|
| [3] |
Fukumoto Y. Takayasu arteritis-associated pulmonary hypertension[J]. Eur Heart J, 2021, 42(42): 4306–4308. doi: 10.1093/eurheartj/ehab688
|
| [4] |
Jiang X, Zhu Y, Zhou Y, et al. Clinical features and survival in Takayasu's arteritis-associated pulmonary hypertension: a nationwide study[J]. Eur Heart J, 2021, 42(42): 4298–4305. doi: 10.1093/eurheartj/ehab599
|
| [5] |
Lin J, Zhang T, Peng M, et al. Clinical features of pulmonary artery involvement in Takayasu's arteritis and recent advances[J]. Chin J Tuberc Respir Dis, 2021, 44(1): 54–59. doi: 10.3760/cma.j.cn112147-20200316-00349
|
| [6] |
Villon MLFZ, De La Rocha JAL, Espinoza LR. Takayasu arteritis: recent developments[J]. Curr Rheumatol Rep, 2019, 21(9): 45. doi: 10.1007/s11926-019-0848-3
|
| [7] |
Russo RAG, Katsicas MM. Takayasu arteritis[J]. Front Pediatr, 2018, 6: 265. doi: 10.3389/fped.2018.00265
|
| [8] |
Esatoglu SN, Hatemi G. Takayasu arteritis[J]. Curr Opin Rheumatol, 2022, 34(1): 18–24. doi: 10.1097/BOR.0000000000000852
|
| [9] |
Podgórska D, Podgórski R, Aebisher D, et al. Takayasu arteritis-epidemiology, pathogenesis, diagnosis and treatment[J]. J Appl Biomed, 2019, 17(1): 20. doi: 10.32725/jab.2018.005
|
| [10] |
Hellmich B. Treatment of Takayasu arteritis[J]. Z Rheumatol (in German), 2020, 79(6): 532–544. doi: 10.1007/s00393-020-00806-2.
|
| [11] |
Dou J, Gong J, Ma Z, et al. The analysis of the clinical records diagnosed as Takayasu's arteritis with pulmonary vascular involvement[J]. Chin J Tuberc Respir Dis, 2016, 39(8): 603–607. doi: 10.3760/cma.j.issn.1001-0939.2016.08.011
|
| [12] |
Yang J, Peng M, Shi J, et al. Pulmonary artery involvement in Takayasu's arteritis: diagnosis before pulmonary hypertension[J]. BMC Pulm Med, 2019, 19(1): 225. doi: 10.1186/s12890-019-0983-7
|
| [13] |
He Y, Lv N, Dang A, et al. Pulmonary artery involvement in patients with Takayasu arteritis[J]. J Rheumatol, 2020, 47(2): 264–272. doi: 10.3899/jrheum.190045
|
| [14] |
Kawai T, Yamada Y, Tsuneda J, et al. Pleural effusion associated with aortitis syndrome[J]. Chest, 1975, 68(6): 826–828. doi: 10.1378/chest.68.6.826
|
| [15] |
Achari V, Prakash S. Takayasu's disease presenting with pain chest, prolonged pyrexia and pleural effusion[J]. J Assoc Physicians India, 2005, 53: 722–724. https://pubmed.ncbi.nlm.nih.gov/16398084/
|
| [16] |
Schattner A, Klepfish A. Left pleural effusion and fever of unknown origin-a clue to thoracic arterial pathology[J]. J Gen Intern Med, 2012, 27(8): 1084–1087. doi: 10.1007/s11606-012-2008-6
|
| [17] |
Gui X, Cao M, Liu Y, et al. Unilateral pleural effusion as first manifestation in Takayasu arteritis: a case report and review of literature[J]. Chin J Tuberc Respir Dis, 2016, 39(10): 768–772. doi: 10.3760/cma.j.issn.1001-0939.2016.10.006
|
| [18] |
Liang Y, Zuo Q, Wang T. A case of Takayasu's arteritis with unilateral pleural effusion as the main manifestation and literature review[J]. Acta Univ Med Nanjing (Nat Sci) (in Chinese), 2018, 38(2): 266–268. doi: 10.7655/NYDXBNS20180228.
|
| [19] |
Light MD, Richard W. Plural disease[M]. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013: 128–129.
|
| [20] |
Na MJ. Diagnostic tools of pleural effusion[J]. Tuberc Respir Dis, 2014, 76(5): 199–210. doi: 10.4046/trd.2014.76.5.199
|
| [1] | Vishal P. Dubey, Varun P. Sureja, Dharmeshkumar B. Kheni. Efficacy evaluation of standardized Rheum rhaponticum root extract (ERr 731®) on symptoms of menopause: A systematic review and meta-analysis study[J]. The Journal of Biomedical Research, 2024, 38(3): 278-286. DOI: 10.7555/JBR.37.20230219 |
| [2] | Leonid N. Maslov, Natalia V. Naryzhnaya, Sergey V. Popov, Alexandr V. Mukhomedzyanov, Ivan A. Derkachev, Boris K. Kurbatov, Andrey V. Krylatov, Feng Fu, Jianming Pei, Vyacheslav V. Ryabov, Evgenii V. Vyshlov, Svetlana V. Gusakova, Alla A. Boshchenko, Akpay Sarybaev. A historical literature review of coronary microvascular obstruction and intra-myocardial hemorrhage as functional/structural phenomena[J]. The Journal of Biomedical Research, 2023, 37(4): 268-289. DOI: 10.7555/JBR.37.20230021 |
| [3] | Pavan Kumar Dhanyamraju, Trupti N. Patel. Melanoma therapeutics: a literature review[J]. The Journal of Biomedical Research, 2022, 36(2): 77-97. DOI: 10.7555/JBR.36.20210163 |
| [4] | Zhang Lei, McLeod Stephanie T., Vargas Rodolfo, Liu Xiaojian, Young Dorthy K., Dobbs Thomas E.. Subgroup comparison of COVID-19 case and mortality with associated factors in Mississippi: findings from analysis of the first four months of public data[J]. The Journal of Biomedical Research, 2020, 34(6): 446-457. DOI: 10.7555/JBR.34.20200135 |
| [5] | Omofoye Oluwaseun A., Binello Emanuela. Intraventricular metastases from rectal carcinoma: case report and literature review[J]. The Journal of Biomedical Research, 2020, 34(4): 318-322. DOI: 10.7555/JBR.33.20180133 |
| [6] | Christopher J. Danford, Zemin Yao, Z. Gordon Jiang. Non-alcoholic fatty liver disease: a narrative review of genetics[J]. The Journal of Biomedical Research, 2018, 32(6): 389-400. DOI: 10.7555/JBR.32.20180045 |
| [7] | Arjang Ahmadpour, Amir Goodarzi, Darrin J. Lee, Ripul R. Panchal, Kee D. Kim. Cervical spine fractures in osteopetrosis: a case report and review of the literature[J]. The Journal of Biomedical Research, 2018, 32(1): 68-76. DOI: 10.7555/JBR.32.20170055 |
| [8] | Yan Zhu, Meining Yu, Luyao Ma, Hai Xu, Fanghong Rose Li. Choriocarcinoma-associated pulmonary thromboembolism and pulmonary hypertension: a case report[J]. The Journal of Biomedical Research, 2016, 30(3): 243-247. DOI: 10.7555/JBR.30.20140062 |
| [9] | Dongsheng Zhao, Qing Zhang, Jingping Lu, Gang Zhang, Huihe Lu, Jianfei Huang, Qijun Shan. Sick sinus syndrome associated with hypopituitarism: a case report and literature review[J]. The Journal of Biomedical Research, 2014, 28(5): 429-432. DOI: 10.7555/JBR.28.20130099 |
| [10] | Hailong Cao, Yanhu Wu, Jinfu Zhu, Yijiang Chen. Familial cardiac myxoma with multifocal recurrences: a case report and review of the literature[J]. The Journal of Biomedical Research, 2011, 25(5): 368-372. DOI: 10.1016/S1674-8301(11)60049-3 |
| Author | Kawai et al[14] | Achari et al[15] | Schattner et al[16] | Gui et al[17] | Liang et al[18] |
| Gender | Male | Female | Female | Female | Female |
| Age (years) | 32 | 32 | 35 | 58 | 72 |
| Physical examination | Pulselessness; Vascular murmur | _ | Vascular murmur; Splenomegaly | Vascular murmur; Pleural effusion signs; Upper limb blood pressure cannot be measured. | Pleural effusion signs |
| Laboratory and imaging examinations | |||||
| Hb (g/dL) | – | 9.2 | 9.1–7.6 | 12.1 | 12.4 |
| CRP (mg/L) | 3+ | – | 102 | 42 | 8.68 |
| ESR (mm/h) | >100 | 60 | 140 | 23 | 23 |
| Autoimmunity | Negative | Negative | Anti-smooth muscle antibodies (+) | Negative | Anti-PM-Sclanmibody (±) |
| Pleural effusion | Left; Exudative; Small amount; Recurring with disease activity | Left; Exudative | Left; Small amount; Exudative | Left; Massive; Bloody | Left; Massive; Exudative |
| Involved narrowed or occluded arteries | Subclavicul bilaterally | Right subclavian; Left coronary artery | Proximal aorta; Right carotid | Subclavicul bilaterally; Right upper pulmonary | Upper and lower left pulmonary artery |
| Treatment | Misdiagnosed as tuberculosis, pleural effusion recurring along with irregular corticosteroids application, and after TA diagnosis, corticosteroids given again. | Prednisolone 60 mg daily with methotrexate 2.5 mg weekly increasing the dose to 7.5 mg weekly a month. | Prednisone 50 mg/day and methotrexate 7.5 mg/week tapered over months to 5 mg/day prednisone and aspirin. | Methylprednisolone 80 mg, 1 time/8 hours, IV, gradually reduced to prednisone 20 mg, 2 times/day orally, cyclophosphamide 0.4 g IV, with torasemide and other symptomatic supportive treatment. | Methylprednisolone 40 mg qd×7 days, intravenous drip, gradually reduced to prednisone acetate tablets 30 m/day orally and hydroxychloroquine sulfate 0.2 g bid. |
| Hb: hemoglobin; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; IV: intravenous; qd: once a day; bid: twice a day. | |||||