| Citation: | Omofoye Oluwaseun A., Binello Emanuela. Intraventricular metastases from rectal carcinoma: case report and literature review[J]. The Journal of Biomedical Research, 2020, 34(4): 318-322. DOI: 10.7555/JBR.33.20180133 |
Cerebral metastases are the most commonly encountered neoplasm of the brain, with its total number diagnosed annually more than that of all the other intracranial tumors together. An estimated 24%–45% of all cancer patients in the United States have brain metastases, and the incidence of cerebral metastases has continued to increase over the last few decades. Intraventricular tumors account for only about 10% of central nervous system (CNS) tumors, and intraventricular metastases comprise an estimated 6% of them[1]. A recently published study showed a 0.1% rate of having brain metastasis during the initial presentation of patients with colorectal cancer, and a 1.2% chance of developing brain metastases during an almost 4-year follow-up period[2]. Intraventricular metastases are a rare occurrence, particularly from a primary colorectal malignancy. There have only been four previously reported cases of intraventricular colon cancer metastases[1,3–5].
Since its initial description in 1963, the Ommaya reservoir has been an important tool in oncologic management, specifically for cerebrospinal fluid (CSF) sampling and administration of intrathecal chemotherapeutic agents for CNS malignancies. Several modifications of the original Ommaya reservoir, including novel technique variations, have been described. These include various forms of image guidance, neuroendoscopy, fluoroscopy, and frame-based or frameless stereotactic adjuncts. Here, we report a case of a 72-year-old male patient with intraventricular metastases from rectal carcinoma.
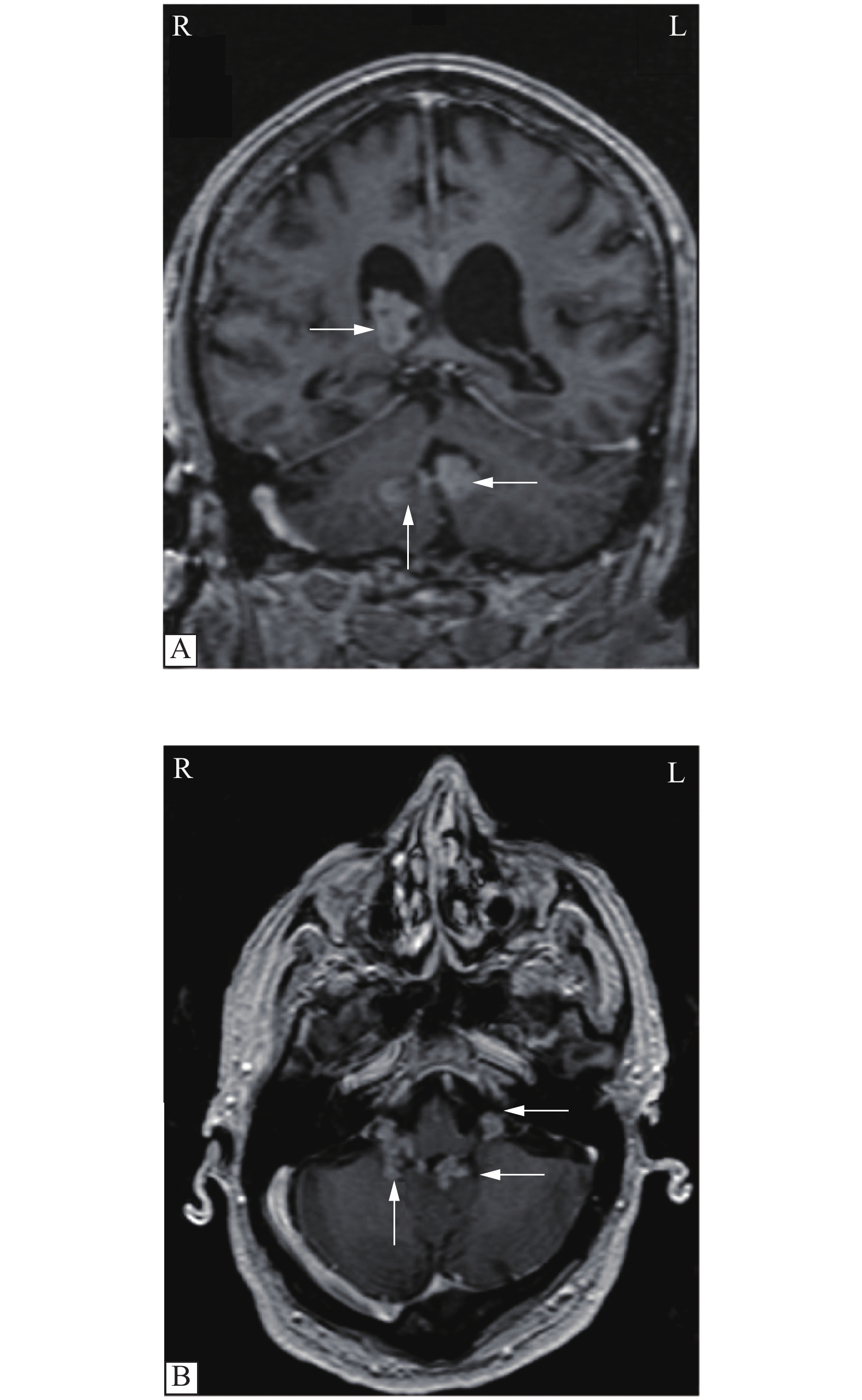
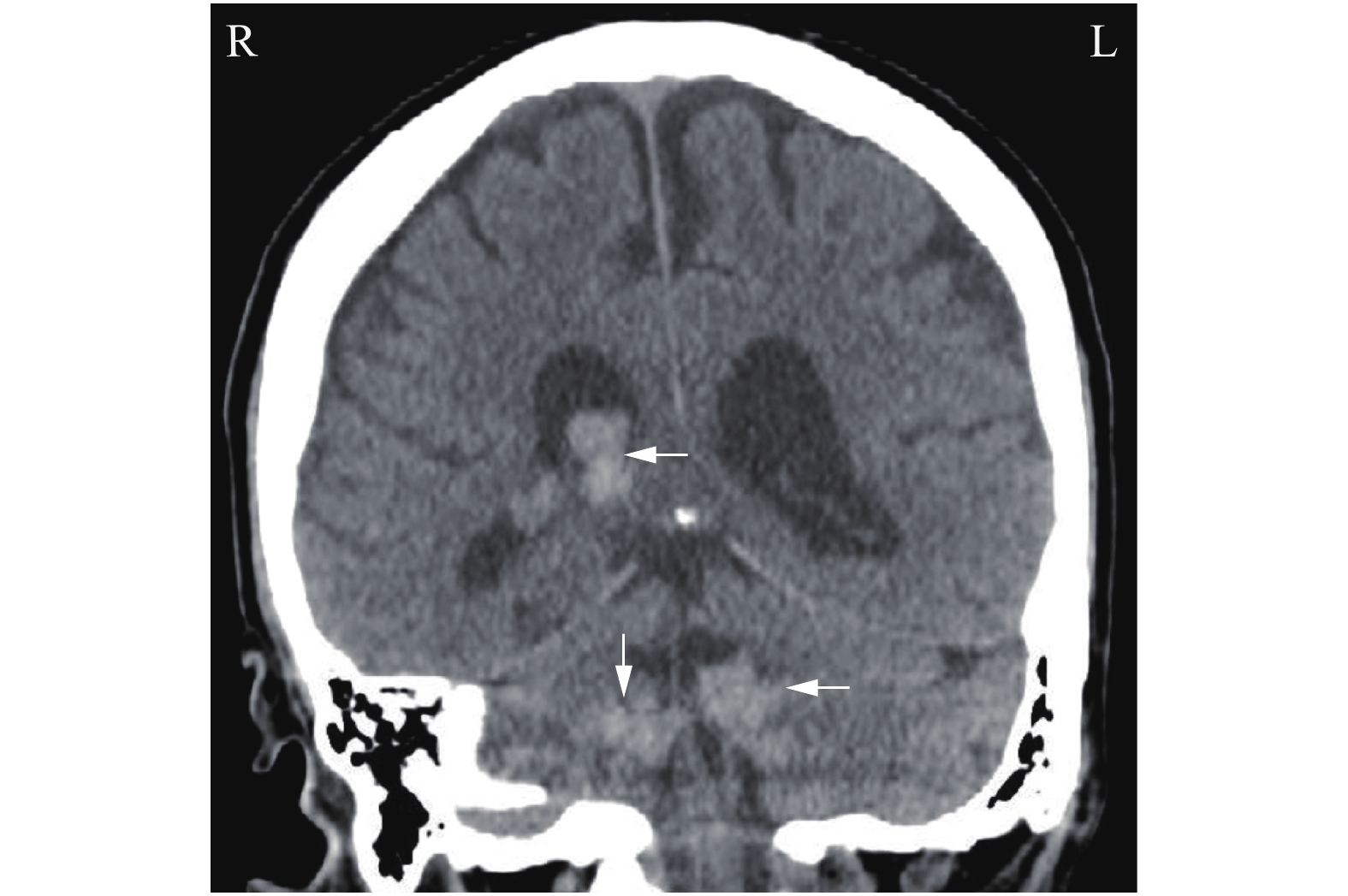
A 72-year-old male, who has been in generally good health for 50 years, presented to an outside hospital with symptoms of hoarseness, dysphagia, headaches, dizziness and chronic diarrhea for a few weeks. His head computed tomography (CT) scan revealed multiple intraventricular lesions (Fig. 1), and he was transferred to our hospital for evaluation. A magnetic resonance imaging (MRI) of his brain confirmed the previously noted enhanced intraventricular lesions within the right lateral ventricle, the fourth ventricle and bilateral foramen of Luschka (Fig. 2). An oncologic workup ensued and a CT of the chest, abdomen, and pelvis revealed thickening of the rectum with surrounding fat stranding and a heterogeneous mass in the right cardiophrenic region (Fig. 3). The patient underwent a flexible sigmoidoscopy with rectal biopsies showing a moderately differentiated invasive adenocarcinoma. He also underwent a CT-guided biopsy of the anterior mediastinal mass, which was diagnosed as a World Health Organization (WHO) grade AB thymoma and was thought to be an incidental diagnosis unrelated to his metastatic rectal adenocarcinoma. The patient's neurological functions were assessed intact with no deficits, but he continued to have progressive dysphagia possibly due to the thymoma impinging on his vocal cords. As opposed to performing an invasive craniotomy for resection of the patient's intraventricular lesions, we decided to proceed with a less invasive intraventricular Ommaya reservoir placement. This decision was predicated by the fact that the patient had multiple ventricular involvement, a potential need for intrathecal chemotherapy and the possibility of developing hydrocephalus. Typically, the Ommaya reservoir placement would be performed under general anesthesia, but the anesthesia team had concerns about intubating the patient in the setting of vocal cord compression from his thymoma. Due to these concerns, the procedure was performed under local anesthesia and monitored airway care rather than general anesthesia.
The patient was slightly sedated under monitored anesthesia care. A total of 0.5 mg remifentanil was administered intravenously at 0.05 to 0.075 μg/kg/minute, 100 μg of fentanyl was used intravenously for sedation, and 6 mL of 1% lidocaine with 1:100 000 epinephrine was injected subcutaneously for local anesthesia. The patient was then positioned supine on a donut head-fixator and the AxiEM electromagnetic neuronavigation system (Medtronic, Louisville, USA) was registered with great accuracy. A trajectory was planned to encompass all the catheter holes in the ventricular system, to avoid affecting the parenchyma. Incision was made at the right frontal Kocher's point and the Ommaya reservoir was placed under navigation with no complications. The reservoir was noted to fill with clear CSF and pumped easily. CSF was sent for cytology and carcinoembryonic antigen (CEA). The patient remained neurologically and hemodynamically stable throughout the procedure.
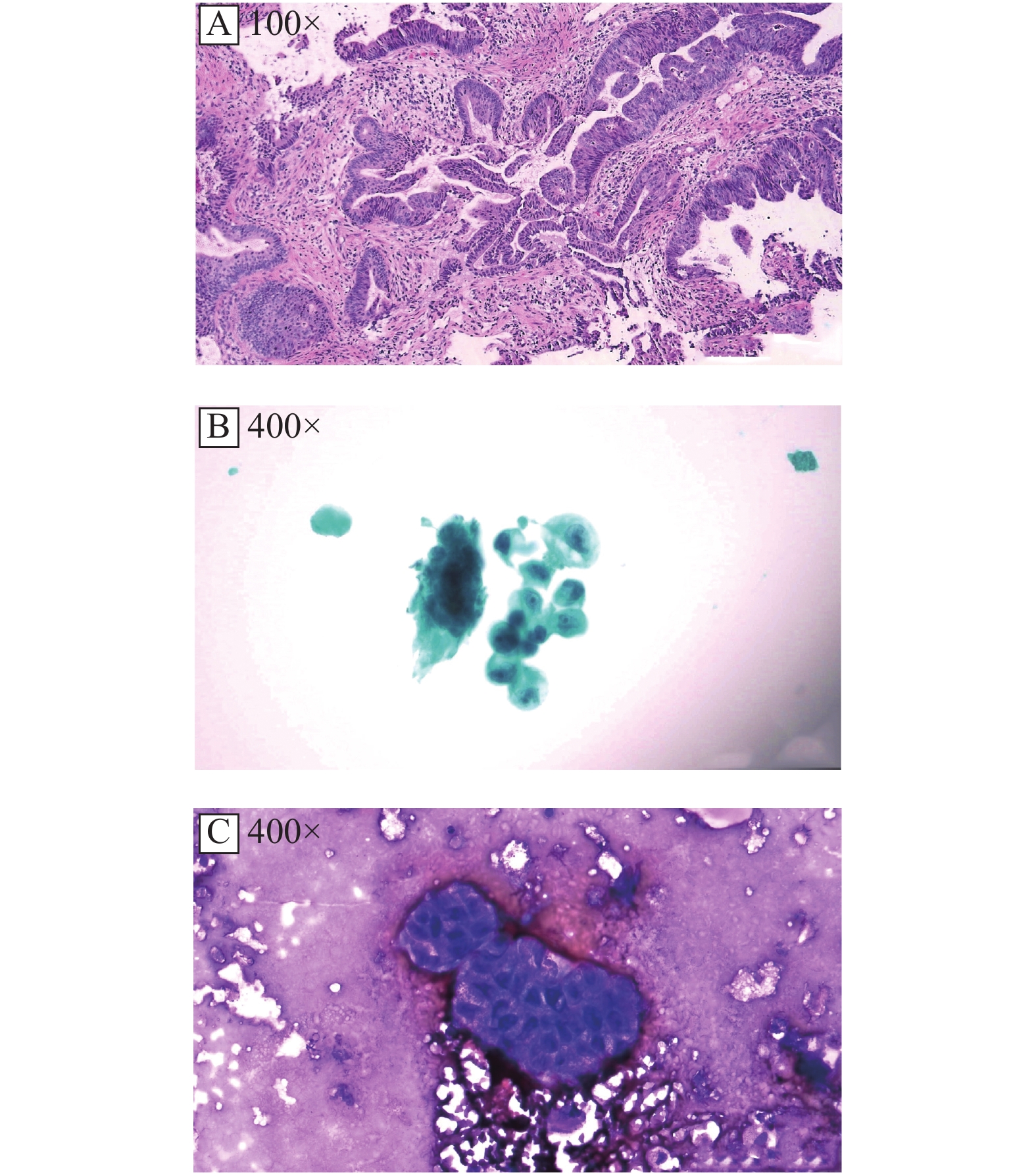
Microscopic examination of the rectal mass demonstrated a moderately differentiated invasive adenocarcinoma arising in tubular adenoma with high grade-dysplasia (Fig. 4A). The tumor was negative for BRAF V600E mutation and KRAS codon 12/13 mutation. Immunohistochemical staining of the tumor showed abnormal loss of expression of MSH2/MSH6 proteins with retained expression of MLH1/PMS2 proteins, suggestive of Lynch syndrome.
CSF cytology was positive for malignancy and consistent with adenocarcinoma (Fig. 4B and C). CSF CEA was also positive, with a level of 2 355.4 ng/mL, while the serum CEA was 13.4 ng/mL, thereby ruling out passive transfer from serum. The results of CSF cytology and CEA tests confirmed the intra-ventricular lesions to be distant metastases of a primary rectal adenocarcinoma.
The patient was neurologically intact postoperatively, only complaining of mild incisional pain. CT scan of the head demonstrated adequate placement of the catheter in the body of the lateral ventricle and small expected intraventricular air. Two weeks postoperatively, the patient underwent whole brain radiation and received 3000 cGy in 10 fractions. Two weeks after that, he was also treated with palliative radiation to the rectum, 3000 cGy in 10 fractions. His clinical course was complicated by aspiration pneumonia, treated with two separate courses of antibiotics, and pulmonary embolism, treated with prophylactic anticoagulation with a consideration of the risk of intracranial hemorrhage from therapeutic anticoagulation. The initial nasogastric tube feeding was replaced by total parenteral nutrition because a jejunostomy tube was unable to be placed surgically due to site obstruction by colon distention from stool and air. Due to poor performance status, he was not a candidate for systemic chemotherapy, rectal surgery or stenting. Given all this, family decided to pursue hospice care. Six weeks postoperatively, he was discharged to a hospice facility where he expired 3 days later.
The overall incidence of cerebral metastases continues to increase due to a variety of factors. Suggested reasons include the increasing length of survival of cancer patients due to improved treatment and management of systemic cancers, as well as earlier diagnosis due to the easy availability of imaging modalities. Other proposed reasons include the fact that many chemotherapeutic agents do not cross the blood-brain barrier (BBB), thereby making the brain a haven for metastases, and paradoxically, some chemotherapeutic agents may transiently weaken the BBB, allowing CNS seeding with tumor[6].
Intraventricular or choroid plexus metastases, particularly of colorectal origin, are a very rare occurrence. However, as the prevalence of systemic cancers continues to rise, there will likely be a corresponding increase in their diagnosis over time. Metastases from cancers of the gastrointestinal tract have not only been described in the brain, but also in head and neck regions such as the scalp. A recent systematic review by Paolino et al noted gastrointestinal cancers account for 24.4% of scalp metastases, five cases of which were secondary to rectal carcinoma[7]. To our knowledge, there have only been four reported cases of intraventricular or choroid plexus metastases from a colon cancer origin, and no prior reports of one from a primary rectal cancer up to this point (Table 1)[1,3–5]. All the cases described in this study or others have been secondary to the histologic diagnosis of adenocarcinoma. Patient age, sex, number of lesions and time from initial diagnosis to intraventricular metastasis was reported in all but one of the four published cases of intraventricular or choroid plexus metastases from a colon cancer origin. The male to female ratio was 2 to 1. The mean age at diagnosis was 58, younger than our patient who was 72 years old at diagnosis. The mean time from initial diagnosis of colon cancer to intraventricular metastases was 4.7 years, while the patient in this study presented with concurrent primary rectal cancer and intraventricular metastases. The most common location of metastasis is the lateral ventricle, with only one prior case involving multiple ventricles[4]. MRI of the brain revealed several enhanced intraventricular lesions within the right lateral ventricle, the fourth ventricle and bilateral foramen of Luschka (Fig. 2), making this case the second published report of multi-ventricular metastasis of colorectal origin, as well as the first published report of intraventricular metastasis from rectal cancer.
| Case | Author (year) | Age (year)/ sex | Metastasis location | Primary tumor origin | No. of lesions | Diagnosis to metastasis (year) | Histologic diagnosis |
| 1 | Kohno et al (1996)[1] | 45/M | Trigone of L lateral ventricle | Sigmoid colon | Solitary | 3 | Adenocarcinoma |
| 2 | Al-Anazi et al (2000)[4] | 81/M | All ventricles | Colon | Multiple | 8 | Adenocarcinoma |
| 3 | Kitajima et al (2003)[5] | 48/F | Inferior horn of R lateral ventricle | Colon | Solitary | 3 | Adenocarcinoma |
| 4 | Hassaneen et al (2010)[3] | NR | Lateral ventricle | Colon | NR | NR | Adenocarcinoma |
| Current | Omofoye et al (2020) | 72/M | All ventricles | Rectal | Multiple | 0 | Adenocarcinoma |
| F: female; L: left; M: male; NR: not reported; R: right. | |||||||
Differences in the metastatic profile of colon and rectal cancer have been reported in the literature. Qiu et al queried the Surveillance, Epidemiology and End Results Program database for colorectal cancer, and noted a higher incidence of liver metastasis from colon cancer than from rectal cancer, while rectal cancer has a higher incidence of lung and bone metastases than colon cancer[8]. Even though their study showed no difference in the rate of brain metastases from colon or rectal cancer, other studies have reported a higher risk of brain metastases in patients with primary rectal cancer[9]. This difference has been explained by the venous drainage of the rectum which bypasses the liver and goes directly into the inferior vena cava[9]. Lynch syndrome is most frequently caused by mutations in the mismatch repair genes MLH1, MSH2, MSH6, and PMS2, leading to microsatellite instability[10]. This patient's loss of two of these genes is consistent with a diagnosis of Lynch syndrome. However, his diagnosis of a WHO grade AB thymoma is not classically seen in Lynch syndrome, which is more commonly associated with colorectal cancer, gastric cancer, transitional carcinoma of the ureter and renal pelvis, glioblastoma, and in females, endometrial and ovarian cancers. Interestingly, there has been one published report of a stage IB malignant thymoma in a Lynch syndrome patient with three synchronous adenocarcinomas of the colon and multiple other cancers[10]. Thymomas have been associated with other tumor types, most notably gastrointestinal cancers. It is unclear whether its occurrence in a Lynch syndrome patient such as this case, or as described by Tampellini et al[10], is directly or indirectly secondary to the same genomic instability mechanism responsible for their correlative colorectal cancers.
As the management of patients with systemic cancers continues to improve with medical discoveries and technological innovation, progressively older patients will have to be managed with therapies, often currently considered too aggressive for them. Surgical and anesthetic management will have to be modified to accommodate patients that have been classically considered non-surgical candidates. Due to this patient's thymoma and compression of his airway, he was deemed as an unsuitable candidate for intubation and general anesthesia for a more invasive resective surgery. After an intraventricular biopsy was considered, it was decided to proceed with an awake placement of Ommaya reservoir. This provided a strategy not only for diagnosis via CSF sampling, but also for possible treatment with intrathecal chemotherapy and CSF access if hydrocephalus were to develop. Awake neurological surgeries such as craniotomies for tumor resection and deep brain stimulation are well-established surgical modalities. But there have been few reports of Ommaya reservoir placements under local anesthesia. This report demonstrates that awake placement of Ommaya reservoir with AxiEM electromagnetic neuronavigation is a viable alternative for selected oncologic patients with intraventricular malignancy or metastases, who may not be candidates for general anesthesia or resective surgery.
Intraventricular or choroid plexus metastases are a very rare occurrence, particularly from primary colorectal cancer. Although rare, this diagnosis should always be considered in the differential for solitary or multiple intraventricular lesions. CSF sampling is a useful alternative to intraventricular biopsy for diagnosis of intraventricular metastases. Awake placement of Ommaya reservoir is a safe option in the management of patients with intraventricular metastases, especially those who cannot undergo general anesthesia.
| [1] |
Kohno M, Matsutani M, Sasaki T, et al. Solitary metastasis to the choroid plexus of the lateral ventricle. Report of three cases and a review of the literature[J]. J Neuro-oncol, 1996, 27(1): 47–52. doi: 10.1007/BF00146083
|
| [2] |
Nozawa H, Ishihara S, Kawai K, et al. Brain metastasis from colorectal cancer: predictors and treatment outcomes[J]. Oncology, 2017, 93(5): 309–314. doi: 10.1159/000478661
|
| [3] |
Hassaneen W, Suki D, Salaskar AL, et al. Surgical management of lateral-ventricle metastases: report of 29 cases in a single-institution experience[J]. J Neurosurg, 2010, 112(5): 1046–1055. doi: 10.3171/2009.7.JNS09571
|
| [4] |
Al-Anazi A, Shannon P, Guha A. Solitary metastasis to the choroid plexus. Case Illustration[J]. J Neurosurg, 2000, 92(3): 506. doi: 10.3171/jns.2000.92.3.0506
|
| [5] |
Kitajima K, Morita M, Morikawa M, et al. Choroid plexus metastasis of colon cancer[J]. Magn Reson Med Sci, 2003, 2(3): 155–158. doi: 10.2463/mrms.2.155
|
| [6] |
Greenberg MS. Handbook of neurosurgery[M]. 8th ed. New York, NY: Thieme, 2016.
|
| [7] |
Paolino G, Pampena R, Grassi S, et al. Alopecia neoplastica as a sign of visceral malignancies: a systematic review[J]. J Eur Acad Dermatol Venereol, 2019, 33(6): 1020–1028. doi: 10.1111/jdv.15498
|
| [8] |
Qiu MZ, Hu JM, Yang DJ, et al. Pattern of distant metastases in colorectal cancer: a SEER based study[J]. Oncotarget, 2015, 6(36): 38658–38666.
|
| [9] |
Hugen N, van de Velde CJH, de Wilt JHW, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype[J]. Ann Oncol, 2014, 25(3): 651–657. doi: 10.1093/annonc/mdt591
|
| [10] |
Tampellini M, Alabiso I, Sculli CM, et al. Stage IB malignant thymoma in a Lynch syndrome patient with multiple cancers: response to incidental administration of oxaliplatin and 5-fluorouracil[J]. J Chemother, 2006, 18(4): 433–436. doi: 10.1179/joc.2006.18.4.433
|
| 1. | Demir MK, Özdamarlar U, Yılmaz B, et al. Magnetic Resonance Imaging of Unusual Neoplasms Related to Foramen of Luschka: A Review for Differential Diagnosis. Indian J Radiol Imaging, 2022, 32(1): 71-80. DOI:10.1055/s-0042-1743113 |
| 2. | Li Y, He L, Wang Y, et al. N6-methyladenosine methyltransferase KIAA1429 elevates colorectal cancer aerobic glycolysis via HK2-dependent manner. Bioengineered, 2022, 13(5): 11923-11932. DOI:10.1080/21655979.2022.2065952 |
| Case | Author (year) | Age (year)/ sex | Metastasis location | Primary tumor origin | No. of lesions | Diagnosis to metastasis (year) | Histologic diagnosis |
| 1 | Kohno et al (1996)[1] | 45/M | Trigone of L lateral ventricle | Sigmoid colon | Solitary | 3 | Adenocarcinoma |
| 2 | Al-Anazi et al (2000)[4] | 81/M | All ventricles | Colon | Multiple | 8 | Adenocarcinoma |
| 3 | Kitajima et al (2003)[5] | 48/F | Inferior horn of R lateral ventricle | Colon | Solitary | 3 | Adenocarcinoma |
| 4 | Hassaneen et al (2010)[3] | NR | Lateral ventricle | Colon | NR | NR | Adenocarcinoma |
| Current | Omofoye et al (2020) | 72/M | All ventricles | Rectal | Multiple | 0 | Adenocarcinoma |
| F: female; L: left; M: male; NR: not reported; R: right. | |||||||