| Citation: | Rampes Sanketh, Ma Daqing. Hepatic ischemia-reperfusion injury in liver transplant setting: mechanisms and protective strategies[J]. The Journal of Biomedical Research, 2019, 33(4): 221-234. DOI: 10.7555/JBR.32.20180087 |
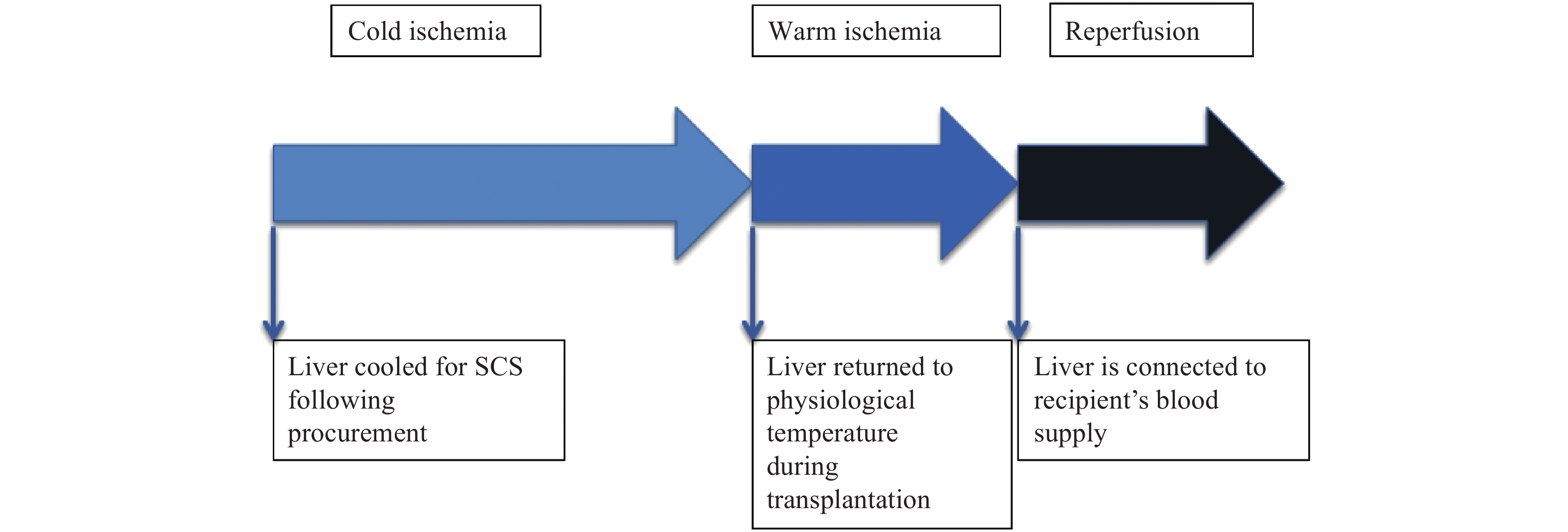
Hepatic ischemia-reperfusion injury (IRI) is a pathological process that involves ischemia-mediated cellular damage followed by a paradoxical exacerbation upon reperfusion of the liver. Hepatic IRI can be classified into two distinct types: warm and cold IRI, which share a similar underlying pathophysiology, albeit with differences especially in the clinical setting[1]. Warm IRI is initiated by hepatocellular damage and occurs during liver transplantation surgery, shock and trauma where there may be a transient fall in blood flow to the liver[2]. Cold IRI is unique to the setting of liver transplantation and is initiated by hepatic sinusoidal endothelial cells and disruption of the microcirculation[1,3]. It occurs during cold storage of the organ prior to transplantation. Both types of IRI are associated with a sterile local, innate immune response[4–5]. Hepatic IRI leads to raised liver enzymes, biliary strictures and graft dysfunction[6]. Hepatic IRI increases the rate of acute and chronic rejection, and is estimated to be responsible for 10% of early organ failure[7– 8]. Hepatic IRI in some cases can lead to multi-organ dysfunction syndrome (MODS) or systemic inflammatory response syndrome (SIRS), both of which have high rates of mortality and morbidity[9].
Hepatic IRI is of increasing importance in the current national shortage of donor organ supply. Liver transplantation is the standard treatment for patients with end-stage liver failure or primary hepatic malignancy[10]. In the UK, the number of patients on the waiting list for a liver transplant has close to be double in the last decade, and around 15% to 20% of these patients will die while waiting for a transplant[11]. Living donor transplantation is a potential way to address the shortfall of organ donors, however, there are concerns about the serious complications and donor mortality of this procedure. About 3% of living donors donate a lobe of their liver[12]. The main focus of addressing the organ shortage has been on using expanded criteria donor (ECD) organs such as those from older, steatotic, cardiac arrest and/or brain death donors. These ‘marginal’ organs are more susceptible to hepatic IRI[10,13]. For these reasons hepatic IRI has been the subject of much research over the past decade to identify the pathophysiology and design targeted therapies to alleviate its effects. In this review, we provide a summary of the pathophysiology and report on different protective strategies for hepatic IRI.
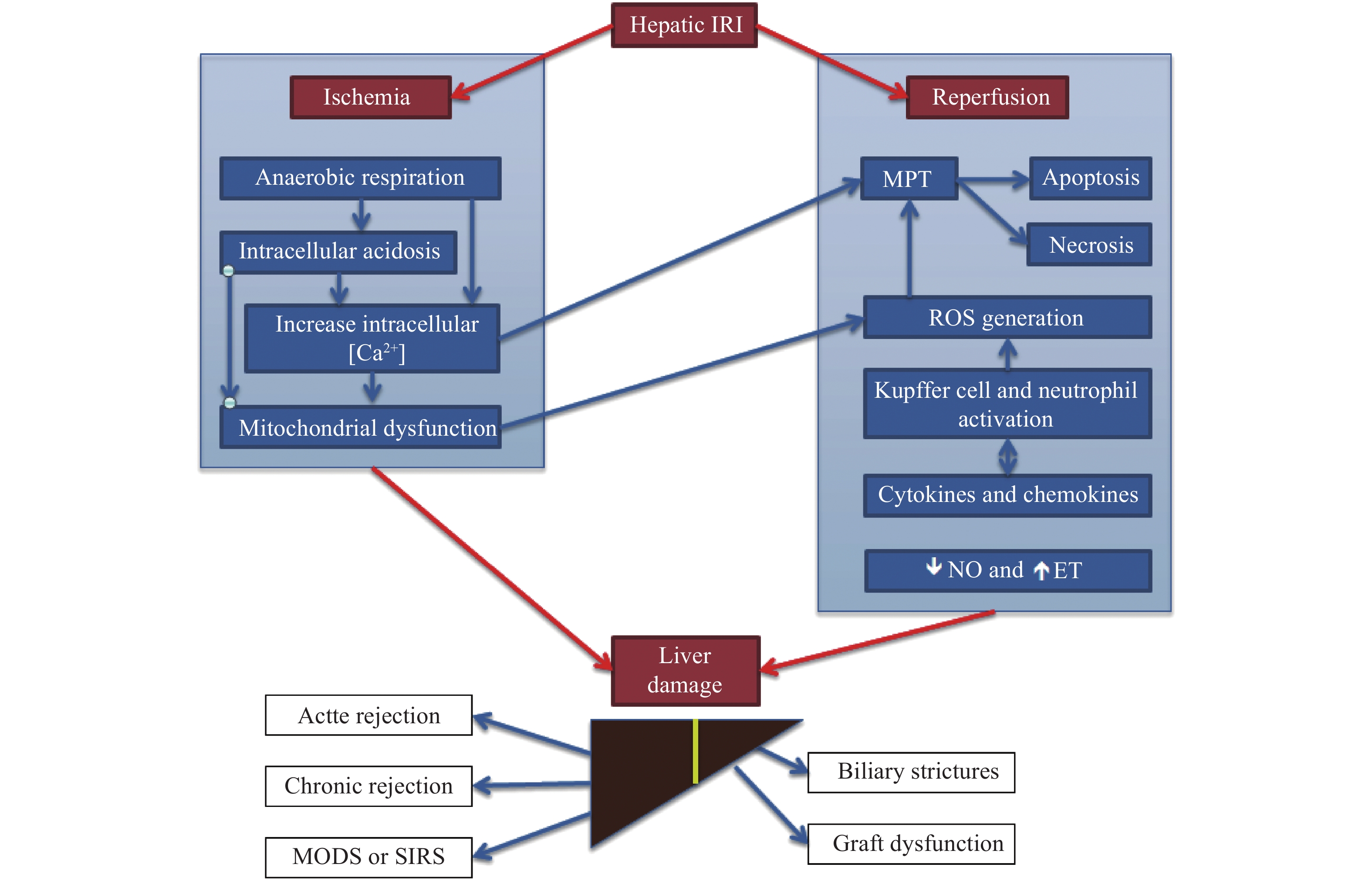
Hepatic IRI is a complex process, which involves many different cell types, the interplay of numerous signaling pathways. Despite being the subject of intense research over the last decade, the mechanism of hepatic IRI is still poorly understood, partially due to the complexity of the process. However, two distinct phases have been identified (Fig. 1). First, the ischemic insult causes functional changes, which facilitate cellular injury[14]. Second, reperfusion of the liver exacerbates the initial injury, which can further be divided into two phases: an early phase which lasts 2 hours after reperfusion and a late phase which lasts 6 to 48 hours after reperfusion[15–16]. The early phase of reperfusion is due to the activation of Kupffer cells (KCs) and sinusoidal endothelial cells (SECs) and the resultant reactive oxygen species (ROS) generation[16–17]. The late phase of reperfusion is caused by the infiltration of neutrophils and CD4+ T-lymphocytes, which release proteases and other cytotoxic enzymes that promote cellular degradation[17–19]. Hepatic IRI can have global consequences, impacting on many remote organs including the: lungs, kidneys, intestines, pancreas and adrenal glands and leading to MODS[20]. The key processes and factors involved in hepatic IRI are: oxidative stress, anaerobic metabolism, nitrous oxide (NO), KCs and neutrophils, mitochondria, intracellular calcium overload, cytokines and chemokines (Fig. 2).
Ischemia results in decreased oxidative phosphorylation, a switch from aerobic to anaerobic metabolism and a fall in ATP production in hepatocytes, SECs and KCs[21]. Increased anaerobic metabolism in hepatocytes leads to intracellular acidosis[22]. The increased intracellular hydrogen ion (H+) concentration leads to increased activity of the sodium (Na+)/H+ exchanger, which results in increased intracellular Na+ concentration. Decreased activity of the ATP-dependent Na+/potassium (K+) exchanger, further increases the concentration of intracellular Na+ resulting in cellular swelling and death[23]. This results in narrowing of the sinusoidal lumen and reduction in microcirculatory blood flow. ATP depletion has a key role in the culmination of necrosis, as illustrated by the attenuation of cell death in SECs and hepatocytes administered glycolytic substrates[24]. Acidosis acts to limit cell damage by suppressing the activity of proteolytic enzymes and phospholipases and by limiting the formation of mitochondrial permeability transition (MPT) pores[25]. Upon reperfusion, the pH of the affected tissue returns to normal, which activates pH-dependent enzymes and results in increased apoptosis and necrosis[26].
Calcium (Ca2+) levels are tightly regulated to maintain a low intracellular Ca2+ concentration. Ca2+ homeostasis is reliant on ATP-dependent processes. The increase in intracellular Na+ concentration during ischemia stimulates the Na+/Ca2+ exchanger, which causes an increase in movement of Ca2+ into the cell. Activation of ryanodine receptors in the endoplasmic reticulum (ER) and of transient receptor potential (TRP) channels in the plasma membrane promotes increased cytosolic Ca2+ concentration[27–28]. Additionally, ATP depletion inhibits Ca2+-ATPase channels in the plasma membrane and ER further contributing to intracellular Ca2+ overload. This activates Ca2+-dependent enzymes including phospholipases and calpains and leads to apoptosis[29]. Increased cytosolic Ca2+ concentration stimulates Ca2+ uniporters in the mitochondrial membrane, leading to an increase in mitochondrial Ca2+ concentration[30]. The disruption of Ca2+ homeostasis triggers the formation of MPT pores which results in either apoptosis or necrosis[31]. Calcium channel blockers have been shown to lower the rise in cytosolic Ca2+ levels and reduce cellular damage, which demonstrates the importance of Ca2+ overload in hepatic IRI[32–35].
During hepatic IRI, the increase in intracellular concentrations of Ca2+, Na+ and H+ all contribute to mitochondrial dysfunction and lead to MPT. The formation of MPT pores causes irreversible damage of the affected mitochondria due to depolarization of the mitochondrial membrane[36]. When a small number of hepatocyte mitochondria are affected, they are degraded by the process of mitophagy[37]. Mitophagy is important for cell survival, as damaged mitochondria are responsible for increases in both ATP consumption and ROS generation[38]. As the proportion of mitochondria undergoing MPT increases, cytochrome C is released from mitochondria initiating apoptosis[39]. When the majority of mitochondria undergo MPT, ATP levels plummet, leading to necrosis of hepatocytes[31,40]. MPT is a common pathway leading to necrosis and apoptosis following hepatic IRI, with necrosis being the predominant mechanism of cellular death[40]. The mitochondria are the largest source of tissue ROS generation upon reperfusion in hepatic IRI[41].
ROS are produced as intermediates or by-products of normal physiological reactions such as oxidative phosphorylation, lipid degradation and inflammation. Cells have effective endogenous antioxidant defense systems to combat these intracellular sources of ROS. The liver has high levels of antioxidants including glutathione, catalase, glutathione reductase and superoxide dismutase[42]. Under homeostatic conditions the liver ’s antioxidant system is effective at combating the damaging effects of ROS. However, upon reperfusion of the ischemic liver, elevated levels of ROS such as superoxide (O2–), hydrogen peroxide (H2O2) and hydroxyl radicals (•OH) can be measured, which overwhelm the hepatic antioxidant system leading to oxidative stress[15,42–43]. Although the exact origin of ROS has yet to be elucidated, the main sources are mitochondrial metabolism, xanthine oxide reductase and NADPH oxidase[44–46]. ROS can cause damage through protein oxidation, lipid peroxidation and DNA damage[47]. ROS additionally can damage endothelial cells compromising the microvasculature[48]. ATP depletion, oxidative stress and microcirculatory disturbances initiate apoptosis and necrosis[49–50].
NO and ET have a key role in regulating blood flow within liver sinusoids, through causing contraction of stellate cells. ET causes contraction of stellate cells in a dose-dependent manner whereas NO causes their relaxation[51]. Hepatic IRI may occur due to an imbalance in the ratio of ET to NO. In the first few hours after reperfusion there is an increase in plasma levels of ET and a concomitant fall in plasma levels of NO[52– 53]. NO is synthesized from L-arginine by the enzyme NO synthase (NOS), of which there are several isoforms. Stimulation of inducible NOS (iNOS) worsens hepatic IRI. iNOS knockout mice subjected to hepatic ischemic reperfusion had lower levels of matrix metalloprotease-9 activity and leukocyte migration in the liver[54]. In contrast, endothelial NOS (eNOS) has been shown to be protective in hepatic IRI through counteracting the deleterious effects of ROS on the liver[55]. NO has a plethora of effects including: causing vasodilation, inhibiting platelet aggregation, inhibiting leukocyte adhesion to vascular endothelial cells, and inhibiting caspases to prevent apoptosis[55]. NO also plays a key role in suppressing pro-inflammatory cytokines and regulating innate and adaptive immunity[56–57]. Strategies aimed at boosting endogenous NO production or delivering exogenous NO have been shown to mitigate the effects of hepatic IRI[58–60].
KCs are resident liver macrophages and form the liver sinusoids together with SECs, hepatic stellate cells and dendritic cells. KCs together with neutrophils play a key role in the development of hepatic IRI. Suppression of KCs with gadolinium chloride attenuates hepatic IRI, whereas activation with latex particles during reperfusion has been shown to worsen the injury[61]. In early reperfusion, KCs become activated and produce ROS, which contributes to hepatic IRI as previously described[62]. The complement system has a key role in activating KCs and also in directly lysing hepatocytes contributing to hepatic IRI[63–64]. Activated KCs additionally produce pro-inflammatory cytokines including interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α)[65]. These cytokines stimulate the increased expression of ICAM-1 and VCAM-1 on the surface of hepatocytes and SECs[66–67]. which causes the activation and migration of CD4+ T-cells and neutrophils[68]. Neutrophils bind to ICAM-1 and VCAM-1, and move into the liver parenchyma from the endothelial lumen. The neutrophils secrete matrix metalloproteinases (MMPs), other proteases and ROS which causes liver damage[44]. In rats, anti-ICAM-1 antibodies have attenuated hepatic IRI following orthotopic liver transplantation[69], supporting the important role of neutrophils in hepatic IRI.
Reperfusion of the ischemic liver triggers the release of cytokines responsible for initiating and maintaining an inflammatory response, which causes hepatic IRI[70]. The cytokine cascade begins with an increase in production of IL-12 and IL-23 by hepatic stellate cells and KCs[71]. Neutralization of IL-12 or IL-23 blunts the rise of TNF-α and IFN-γ post reperfusion, resulting in less neutrophil migration and liver damage[72–73]. TNF-α is possibly an important mediator in the inflammatory response during hepatic IRI[74]. TNF-α has several important functions, including inducing synthesis of the chemokine epithelial neutrophil activating protein-78 (ENA-78), upregulating expression of ICAM-1 and VCAM-1 on endothelial cells and activating the transcription factor NF-kβ[75–76]. Neutralization of TNF-α prevents hepatic IRI by suppressing the inflammatory response[74]. IL-1β is another early response cytokine shown to magnify the inflammatory response during IRI. Antagonism of IL-1β has been shown to reduce TNF-α expression and liver damage during hepatic IRI[77]. Chemokines are low molecular weight proteins that stimulate the recruitment of leukocytes. During reperfusion, high levels of ELR+ CXC chemokines are produced, which contributes to the recruitment and activation of neutrophils[72,78]. Neutralization of ELR+ CXC chemokines, attenuates neutrophil accumulation and subsequent liver damage, albeit partially suggesting non-chemokine chemoattractants may play a role in the recruitment of neutrophils such as leukotriene B4[79]. IL-13 in an anti-inflammatory cytokine that inhibits NF-kβ, which has been shown to have a protective role in hepatic IRI by reducing liver damage and increasing liver regeneration[80].
Multiple pharmacological treatments have been trialed in the prevention of hepatic IRI. They are mainly aimed at combating the increased oxidative stress during hepatic IRI or using immunomodulation. Some of these treatments will be briefly reviewed.
Melatonin is a potent endogenous antioxidant synthesized in the pineal gland[81–82]. Melatonin acts directly as a free radical scavenger, as well as indirectly through upregulating the expression of several antioxidant enzymes such as superoxide dismutase, glutathione peroxidase and glutathione reductase[83– 84]. Multiple studies have shown that melatonin attenuates hepatic IRI through the preservation of ATP synthesis and mitochondrial function[85–86]. The addition of melatonin to perfusate in rat models of cold storage improved bile production and maintained hepatic ATP levels during IRI[87].
Ex-vivo preservation of organs prior to transplant shows beneficial effects across a range of conditions and organs[88]. However, there has been limited research into the protection offered in the context of hepatic IRI. A case series of 4 patients who underwent xenon anesthesia during liver transplantation were all found hemodynamically stable with normal liver function 7 days post-operatively[89]. Further research should investigate the role of noble gases in the prevention of hepatic IRI.
N-acetylcysteine (NAC) is a glutathione precursor. NAC has been shown to be effective against hepatic IRI in animal models[90–91]. Multiple clinical trials on patients undergoing liver transplantation, have shown that administration of intravenous NAC is associated with a lower incidence of primary graft dysfunction and improved liver function[91–92]. However, the literature is mixed and some studies fail to show a benefit of intravenous NAC in hepatic IRI[93–94].
P-selectin blockade has been shown to be effective against hepatic IRI[95]. Treatment with a recombinant P-selectin antagonist (rPSGL-Ig) has been shown effective against hepatic IRI, by reducing neutrophil and leukocyte infiltration, and decreasing the production of pro-inflammatory cytokines[96–97]. rPSFL-Ig has been associated with improved survival and hepatic function in a rat model of hepatic IRI[96]. A phase Ⅱ study of rPSGL-Ig in patients undergoing deceased donor whole liver transplantation found a beneficial effect on graft function through lower AST and ALT, however, the results were not statistically significant[98].
Studies have shown that the use of the caspase inhibitor IDN-6556 in models of hepatic IRI results in decreased hepatic apoptosis and injury[99–100]. A phase Ⅱ trial was designed to test whether supplementation of the preservation solution with IDN-6556 was able to reduce hepatic IRI. IDN-6556 was also administered intravenously to the transplant recipients 0.5 μg/kg every 6 hours for 24 or 48 hours. The study found that IDN-6556 lowered apoptosis markers, hepatic injury and delayed graft dysfunction when added only to the storage and flush solutions[101]. Interestingly, when given intravenously the beneficial effect was abrogated, which may be explained by the prevention of neutrophil apoptosis, thus prolonging the inflammatory response[101].
Akt also known as protein kinase B (PKB) is a serine/threonine kinase, which is essential for cell growth and survival[102]. The Akt pathway is activated during hepatic IRI and is protective in nature. Ischemic preconditioning, ischemic post-conditioning, pharmacological treatments and miRNA-based therapies are four strategies to treat hepatic IRI, and they all involve activation of Akt as a key mediator[103]. Therefore Akt is a prime target for the treatment of hepatic IRI and should be explored in human trials. Melatonin is a potential therapeutic, which activates Akt, increases FoxO1 phosphorylation and decreases markers of hepatic injury[104].
AMP-activated protein kinase (AMPK) plays a key role in energy homeostasis within cells, through regulating energy metabolism[105]. Studies have shown that AMPK may provide beneficial effects in hepatic IRI[106]. Administration of AMPK activator AICAR preserved ATP content, reduced hepatocyte apoptosis and mitigated hepatic IRI[106]. Adiponectin displays an AMPK-dependent protective role in hepatic IRI, further supporting AMPK activation as a novel treatment for hepatic IRI[107–108].
Peroxisome proliferator-activated receptor gamma (PPARγ) forms a heterodimer with the retinoid X Receptor (RXR) to either induce or repress gene transcription[109]. In hepatic IRI, PPARγ expression rises, which increases the resistance of hepatocytes to necrosis and apoptosis[110]. Recently FAM3A has been identified as a target gene of PPARγ[111]. FAM3A attenuates ROS generation, represses NF-kβ activation and stimulates Akt signaling pathways, which cumulatively protect against hepatic IRI[112]. PPARγ should therefore be trialed in human hepatic IRI.
miRNAs are a class of small non-coding RNA molecules that downregulate gene expression through different mechanisms. miRNA expression profiles have been shown to be deregulated in hepatic IRI[113–114]. Many miRNAs are involved in hepatic IRI, however, miR-122 is the most highly expressed. Inhibition of miR-122 has been shown to protect against hepatic IRI[112,115]. miR-34a, miR-370 and miR-155 have also been implicated in hepatic IRI[112]. miRNAs could serve as biomarkers for hepatic IRI, and also as targets for treatment.
IPC involves exposing the liver to a brief period of ischemia (5 –15 minutes), usually by portal triad clamping, followed by a longer period of reperfusion (10 –20 minutes), prior to a period of prolonged ischemia. There is strong pre-clinical evidence of the benefits of IPC with numerous experimental and animal studies which show IPC reduces the severity of hepatic IRI by promoting the survival of hepatocytes[116]. IPC has been shown to lower oxidative stress by decreasing mitochondrial ROS production during hepatic IRI[117]. IPC activates heme-oxygenase 1 (HO-1), an antioxidant enzyme, constitutively expressed in endothelial cells, hepatic stellate cells and hepatocytes[118]. Inhibition of HO-1 with protoporphyrin Ⅳ in rat models of IPC worsens hepatic IRI. Autophagy is another mechanism involved in the protection provided by IPC. Livers administered IPC show markers of elevated autophagy, and in rats subjected to IPC, inhibition of autophagy with wortmanin worsens hepatic IRI[119]. During IPC the mild increase in oxidative stress triggers cellular adaptation by reducing the activity of ATP synthase, thus conserving hepatic ATP levels and increasing the tolerance of cells to MPT[120– 121]. IPC selectively induces increased activity of eNOS which is protective against hepatic IRI[122]. Levels of transaminases, namely aspartate transaminase (AST) and alanine transaminase (ALT) are the most reliable biomarkers of liver injury, and are used to show the protective effects of IPC. IPC has been demonstrated to decrease circulating levels of AST and ALT[123].
Despite the favorable reduction in biomarkers for liver damage, the long-term effects on morbidity and mortality have not been so promising. A cochrane review published in 2008 included 5 randomized controlled trials (RCTs) showed no statistically significant differences in mortality, graft function or graft failure of IPC in the context of liver transplantation[124]. A recent review analyzed 9 clinical trials of IPC in liver transplantation and found that 6 trials did not demonstrate any difference between the IPC group compared to the control group[125]. The time of ischemia to the liver has been suggested as a cause for the discrepancy in results of clinical trials examining IPC to date, therefore an optimum time for ischemia should be decided for subsequent trials[126–127].
IPostC occurs after prolonged ischemia to the organ, and involves administering bursts of controlled reperfusion prior to continuous reperfusion within the recipient[128]. IPostC is more clinically relevant than IPC due to the timing of the procedure, since the timing of ischemia particularly in the liver transplantation setting cannot always be predicted. IPC must be delivered before the onset of the ischemia, which is not always possible. The mechanisms responsible for the protection offered by IPC and IPostC appear to be similar (Fig. 3). It is thought that abrupt reperfusion flushes out endogenous protective substances, whereas a controlled, slower reperfusion maintains protective substances within the liver tissue for longer. IPostC causes increased expression of antioxidant enzymes, reduced neutrophil infiltration[129–130] and increased expression of anti-apoptotic enzymes[131]. IPostC additionally has been shown to modify MPT and to upregulate activity of eNOS and iNOS which may explain the favorable effects on the microcirculation[132–133]. Multiple comparison studies reveal that IPostC offers a similar level of protection to IPC[128].
Two clinical trials have used IPostC for liver transplantation. Both trials showed no benefits of IPostC on post-operative liver function tests or long-term mortality and morbidity[134–135]. However, one of the trials showed improved tolerance to hepatic IRI on histological findings, whereas interestingly the other trial showed that IPostC achieved a significant reduction in post-operative AKI[134]. Similar to IPC, calculating an optimal ischemic time may bring favorable impact on the translation of preclinical results into improvements in clinical outcomes for patients.
Machine perfusion has been the subject of much attention in the field of liver transplantation, and current evidence suggests that it will be most beneficial when applied to ECD organs. Machine perfusion aims to allow reconditioning of ECD organs, the testing of organ function and extension of organ preservation[136]. The perfusate used for HMP is similar to that used for static cold storage (SCS) and the perfusion technique is cheaper and simpler than normothermic machine perfusion (NMP). The main limitation of HMP is that data cannot be generated to assess liver function[137–138]. HMP is usually performed between 8 °C and 12 °C, although the optimal temperature is currently up for debate. Lower temperatures have the advantage of depressed metabolic activity, but cause increased viscosity[139]. This necessitates the use of a higher perfusion pressure, which increases the risk of SEC damage[140]. Additional oxygenation of HMP perfusate (HOPE) seems to be the key in preventing hepatic IRI[141]. In contrast to NMP, HOPE decreases the release of ROS during reperfusion[141]. HOPE additionally decreases inflammatory response pathways and causes the complete restoration of mitochondrial ATP status within 2 hours[141–142].
Using HMP in marginal livers from donors after brain death (DBD) showed reduced hospital stay and biliary complications compared to SCS controls, which was achieved without the use of additional oxygenation[143]. Promising data on the use of HOPE for livers from donors after cardiac death (DCD) has resulted in the nationwide adoption of HOPE for all DCD livers in the context of liver transplantation. Comparison of outcomes of these organs with matched SCS controls from the UK and the Netherlands has revealed a lower incidence of biliary complications and superior 1-year graft survival[144]. The first long-term study of the use of HOPE in 50 marginal DCD livers revealed a similar 5-year survival when compared to low risk DBD liver transplantation[145]. Further research will be needed to determine the optimal perfusion route and level of oxygenation for HOPE.
NMP uses a blood-based perfusate to perfuse the liver and maintain physiological metabolism during the preservation period. NMP is typically commenced at the site of organ retrieval, and is maintained for 3 –19 hours during organ transport before transplantation into the recipient[146]. NMP reduces the severity of hepatic IRI. NMP is thought to help maintain a healthy endothelium and replenish hepatic ATP stores, which has been demonstrated in a porcine model[147]. Glycogen repletion of the liver following NMP has been demonstrated in human studies[148]. NMP alters the expression of genes involved in liver regeneration and the control of inflammation[149]. NMP has been shown to be beneficial in extending liver preservation with the longest preservation time close to 19 hours[150]. NMP played a role in the successful transplantation of a marginal liver 26 hours after procurement, through assessing its viability and enabling the transplant to be delayed until exclusion of extra-hepatic malignancy[151]. Extended preservation time afforded by the use of NMP brings about new challenges such as counteracting the prolonged sheer stress red blood cells (RBCs) are exposed to. To overcome this, Hemopure, an acellular haemoglobin-based carrier has been developed and tested in a liver model of NMP. The results showed increased oxygen extraction in Hemopure perfused livers compared with those perfused with RBCs, but no difference was found in markers of cell death[152].
In contrast to HMP, NMP allows the generation of data that can be used to assess the viability of the liver[148,153]. Currently, no objective markers are used to determine viability for liver transplantation and livers are discarded based on the assessment of donor characteristics and gross appearance of the liver[154–155]. Based on pre-clinical experiments, composite criteria to assess viability for transplant was formed, according to macroscopic appearance, vascular flows, lactate clearance and bile production[156]. These criteria were applied to 6 livers (4 DCD and 2 DBD) declined by all UK transplant centres and subjected to NMP. Of these 6 livers, 5 met the viability criteria and were successfully transplanted with immediate liver function, and normalized liver function tests within a month[148]. Other groups have proposed alternate criteria for assessing viability: Watson et al.[157] suggest viability assessment based on bile pH and perfusate transaminases. The ability of the liver to produce alkaline bile has been hypothesized as a marker of acceptable cholangiocyte function. If validated, this marker will allow the prevention of liver transplants with a limited life span[157]. Future liver viability assessment may use metabolomics profiling or microRNA analysis[158–159].
The first clinical study of NMP in 16 DBD livers and 4 DCD livers showed significantly lower peak AST in the first week postoperatively compared to SCS controls, which may indicate a reduction in the severity of hepatic IRI[146]. The first randomized controlled trial by the Consortium of Organ Preservation in Europe (COPE) has recently been completed. Two hundred and twenty two livers were transplanted, of which 121 were NMP and 101 SCS. The study showed that NMP was associated with a 50% lower rate of organ discard and a 50% lower level of AST release, despite 54% longer mean preservation times[160]. There were no significant differences in graft survival, bile duct complications or patient survival, however, the authors acknowledged that larger trials were required to test these outcomes[160]. The increase in utilization and in preservation times may be the greatest strength of NMP, through allowing the transplantation of currently deemed “untransplantable” organs.
Assessing organ viability prior to transplantation, and improving the quality of liver grafts are the two main barriers to the use of marginal livers. NMP may address both of these limitations. It is necessary to determine whether NMP is as effective after a period of SCS, or whether it is required for the full period of organ preservation.
The use of steatotic organs is severely limited by their increased susceptibility to hepatic IRI[161]. Macrovesicular steatosis in 30% or more of hepatocytes has been shown to decrease graft survival 1 year post-transplant by 71%[162]. Animal studies show that high sensitivity of steatotic livers to hepatic IRI can be reversed by “defatting” the livers[163]. NMP has been shown to be capable of reducing macrovesicular steatosis in rat livers[164]. Unpublished data by Bosteon and colleagues show the solubilisation of fat starting within 3 hours and lasting up to 24 hours of NMP, which results in better metabolic parameters and histologic improvement[165]. Further research should investigate whether defatting livers results in improved clinical outcomes in human trials.
NMP is unique in that it allows for treatment of the liver ex vivo during the preservation period. This can be applied in the context of defatting. However, it can also be used to treat the liver with anti-inflammatory drugs, which has been shown to significantly lower the production of AST, IL-6, TNF-α and increase IL-10 compared to the untreated controls[166]. NMP will allow for the delivery of gene-based therapies such as myr-Akt, which induces cytoprotection against hepatic IRI[167]. The addition of mesenchymal stem cells (MSCs) to perfusate may allow the regrowth of damaged organs before transplantation[168].
Hepatic IRI is a pathological process, which involves ischemia-mediated cellular damage exacerbated upon reperfusion. It is estimated to be responsible for 10% of early organ failure in the liver transplant setting. The pathophysiology of hepatic IRI is complex and involves numerous pathways. The key mediators involve ATP depletion, intracellular acidosis, increased intracellular Ca2+ overload, mitochondrial dysfunction, ROS generation, NO and ET, KC and neutrophil activation, chemokines and cytokines. Numerous drugs have been trialed for the prevention of hepatic IRI, some of which have been reviewed in this article. Akt activators, AMPK activators, PPARγ agonists and miRNA-based therapies have all been identified as promising treatment strategies and warrant further clinical research. IPC and IPostC have extensive preclinical evidence supporting their use, however, clinical evidence has been more ambiguous. Therefore, IPC and IPostC warrant further research with optimized protocols to validate their efficacy. The greatest potential in the treatment of hepatic IRI is machine perfusion, specifically NMP, which additionally allows for assessment of organ viability and reconditioning of the organ prior to transplantation. If validated, NMP will allow the use and reconditioning of marginal livers, which will help reduce the national shortage of livers for transplantation. Further clinical research should therefore be directed at NMP and the reconditioning of marginal livers.
This work was supported by British Journal of Anaesthesia Fellowship grant, NIAA, London, UK.
| [1] |
Ikeda T, Yanaga K, Kishikawa K, et al. Ischemic injury in liver transplantation: difference in injury sites between warm and cold ischemia in rats[J]. Hepatology, 1992, 16(2): 454–461. doi: 10.1002/hep.1840160226
|
| [2] |
Jaeschke H. Reperfusion injury after warm ischemia or cold storage of the liver: role of apoptotic cell death[J]. Transplant Proc, 2002, 34(7): 2656–2658. doi: 10.1016/S0041-1345(02)03464-4
|
| [3] |
Huet PM, Nagaoka MR, Desbiens G, et al. Sinusoidal endothelial cell and hepatocyte death following cold ischemia-warm reperfusion of the rat liver[J]. Hepatology, 2004, 39(4): 1110–1119. doi: 10.1002/hep.20157
|
| [4] |
Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation[J]. Transplant Proc, 2005, 37(4): 1653–1656. doi: 10.1016/j.transproceed.2005.03.134
|
| [5] |
Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation[J]. Am J Transplant, 2011, 11(8): 1563–1569. doi: 10.1111/ajt.2011.11.issue-8
|
| [6] |
Pine JK, Aldouri A, Young AL, et al. Liver transplantation following donation after cardiac death: an analysis using matched pairs[J]. Liver Transpl, 2009, 15(9): 1072–1082. doi: 10.1002/lt.v15:9
|
| [7] |
Howard TK, Klintmalm GBG, Cofer JB, et al. The influence of preservation injury on rejection in the hepatic transplant recipient[J]. Transplantation, 1990, 49(1): 103–107. doi: 10.1097/00007890-199001000-00023
|
| [8] |
Fellström B, Aküyrek LM, Backman U, et al. Postischemic reperfusion injury and allograft arteriosclerosis[J]. Transplant Proc, 1998, 30(8): 4278–4280. doi: 10.1016/S0041-1345(98)01412-2
|
| [9] |
Guo WA. The search for a magic bullet to fight multiple organ failure secondary to ischemia/reperfusion injury and abdominal compartment syndrome[J]. J Surg Res, 2013, 184(2): 792–793. doi: 10.1016/j.jss.2012.06.024
|
| [10] |
Wertheim JA, Petrowsky H, Saab S, et al. Major challenges limiting liver transplantation in the United States[J]. Am J Transplant, 2011, 11(9): 1773–1784. doi: 10.1111/j.1600-6143.2011.03587.x
|
| [11] |
Neuberger J. Liver transplantation in the United Kingdom[J]. Liver Transpl, 2016, 22(8): 1129–1135. doi: 10.1002/lt.v22.8
|
| [12] |
NHS Blood and Transplant. Annual activity report[EB/OL]. [2017-02-07]. www.odt.nhs.uk.
|
| [13] |
Singal AK, Guturu P, Hmoud B, et al. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease[J]. Transplantation, 2013, 95(5): 755–760. doi: 10.1097/TP.0b013e31827afb3a
|
| [14] |
Casillas-Ramírez A, Mosbah IB, Ramalho F, et al. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation[J]. Life Sci, 2006, 79(20): 1881–1894. doi: 10.1016/j.lfs.2006.06.024
|
| [15] |
Fan CG, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver[J]. J Mol Med (Berl), 1999, 77(8): 577–592. doi: 10.1007/s001099900029
|
| [16] |
Zwacka RM, Zhou WH, Zhang YL, et al. Redox gene therapy for ischemia/reperfusion injury of the liver reduces AP1 and NF-κB activation[J]. Nat Med, 1998, 4(6): 698–704. doi: 10.1038/nm0698-698
|
| [17] |
Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection[J]. J Gastroenterol Hepatol, 2003, 18(8): 891–902. doi: 10.1046/j.1440-1746.2003.03056.x
|
| [18] |
Mavier P, Preaux AM, Guigui B, et al. In vitro toxicity of polymorphonuclear neutrophils to rat hepatocytes: evidence for a proteinase-mediated mechanism[J]. Hepatology, 1988, 8(2): 254–258. doi: 10.1002/hep.1840080211
|
| [19] |
Li XK, Matin AF, Suzuki H, et al. Effect of protease inhibitor on ischemia/reperfusion injury of the rat liver[J]. Transplantation, 1993, 56(6): 1331–1336. doi: 10.1097/00007890-199312000-00008
|
| [20] |
Nastos C, Kalimeris K, Papoutsidakis N, et al. Global consequences of liver ischemia/reperfusion injury[J]. Oxid Med Cell Longev, 2014, 2014: 906965.
|
| [21] |
Selzner M, Selzner N, Jochum W, et al. Increased ischemic injury in old mouse liver: an ATP-dependent mechanism[J]. Liver Transpl, 2007, 13(3): 382–390. doi: 10.1002/lt.21100
|
| [22] |
Wang D, Dou K, Song Z, et al. The Na(+)/H(+) exchange inhibitor: a new therapeutic approach for hepatic ischemia injury in rats[J]. Transplant Proc, 2003, 35(8): 3134–3135. doi: 10.1016/j.transproceed.2003.10.021
|
| [23] |
Carini R, De Cesaris MG, Splendore R, et al. Alterations of Na+ homeostasis in hepatocyte reoxygenation injury[J]. Biochim Biophys Acta, 2000, 1500(3): 297–305. doi: 10.1016/S0925-4439(99)00114-3
|
| [24] |
Nishimura Y, Romer LH, Lemasters JJ. Mitochondrial dysfunction and cytoskeletal disruption during chemical hypoxia to cultured rat hepatic sinusoidal endothelial cells: the pH paradox and cytoprotection by glucose, acidotic pH, and glycine[J]. Hepatology, 1998, 27(4): 1039–1049. doi: 10.1002/hep.510270420
|
| [25] |
Vairetti M, Richelmi P, Bertè F, et al. Role of pH in protection by low sodium against hypoxic injury in isolated perfused rat livers[J]. J Hepatol, 2006, 44(5): 894–901. doi: 10.1016/j.jhep.2005.08.007
|
| [26] |
Gores GJ, Nieminen AL, Wray BE, et al. Intracellular pH during " chemical hypoxia” in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death[J]. J Clin Invest, 1989, 83(2): 386–396. doi: 10.1172/JCI113896
|
| [27] |
Jiang N, Zhang ZM, Liu L, et al. Effects of Ca2+ channel blockers on store-operated Ca2+ channel currents of Kupffer cells after hepatic ischemia/reperfusion injury in rats[J]. World J Gastroenterol, 2006, 12(29): 4694–4698. doi: 10.3748/wjg.v12.i29.4694
|
| [28] |
Barritt GJ, Chen JL, Rychkov GY. Ca2+-permeable channels in the hepatocyte plasma membrane and their roles in hepatocyte physiology[J]. Biochim Biophys Acta, 2008, 1783(5): 651–672. doi: 10.1016/j.bbamcr.2008.01.016
|
| [29] |
Wang HG, Pathan N, Ethell IM, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD[J]. Science, 1999, 284(5412): 339–343. doi: 10.1126/science.284.5412.339
|
| [30] |
Anderson CD, Pierce J, Nicoud I, et al. Modulation of mitochondrial calcium management attenuates hepatic warm ischemia-reperfusion injury[J]. Liver Transpl, 2005, 11(6): 663–668. doi: 10.1002/lt.20407
|
| [31] |
Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury[J]. Gastroenterology, 2003, 125(4): 1246–1257. doi: 10.1016/S0016-5085(03)01209-5
|
| [32] |
Nauta RJ, Tsimoyiannis E, Uribe M, et al. The role of calcium ions and calcium channel entry blockers in experimental ischemia-reperfusion-induced liver injury[J]. Ann Surg, 1991, 213(2): 137–142. doi: 10.1097/00000658-199102000-00008
|
| [33] |
Hataji K, Watanabe T, Oowada S, et al. Effects of a calcium-channel blocker (CV159) on hepatic ischemia/reperfusion injury in rats: evaluation with selective NO/pO2 electrodes and an electron paramagnetic resonance spin-trapping method[J]. Biol Pharm Bull, 2010, 33(1): 77–83. doi: 10.1248/bpb.33.77
|
| [34] |
Nicoud IB, Knox CD, Jones CM, et al. 2-APB protects against liver ischemia-reperfusion injury by reducing cellular and mitochondrial calcium uptake[J]. Am J Physiol Gastrointest Liver Physiol, 2007, 293(3): G623–G630. doi: 10.1152/ajpgi.00521.2006
|
| [35] |
Pronobesh C, Dagagi AV, Pallab C, et al. Protective role of the calcium channel blocker amlodipine against mitochondrial injury in ischemia and reperfusion injury of rat liver[J]. Acta Pharm, 2008, 58(4): 421–428.
|
| [36] |
Abu-Amara M, Yang SY, Tapuria N, et al. Liver ischemia/reperfusion injury: processes in inflammatory networks—a review[J]. Liver Transpl, 2010, 16(9): 1016–1032. doi: 10.1002/lt.22117
|
| [37] |
Elmore SP, Qian T, Grissom SF, et al. The mitochondrial permeability transition initiates autophagy in rat hepatocytes[J]. FASEB J, 2001, 15(12): 2286–2287. doi: 10.1096/fj.01-0206fje
|
| [38] |
Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy[J]. Arch Biochem Biophys, 2007, 462(2): 245–253. doi: 10.1016/j.abb.2007.03.034
|
| [39] |
Zhao KS, Zhao GM, Wu DL, et al. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury[J]. J Biol Chem, 2004, 279(33): 34682–34690. doi: 10.1074/jbc.M402999200
|
| [40] |
Kim JS, Qian T, Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes[J]. Gastroenterology, 2003, 124(2): 494–503. doi: 10.1053/gast.2003.50059
|
| [41] |
Sastre J, Serviddio G, Pereda J, et al. Mitochondrial function in liver disease[J]. Front Biosci, 2007, 12: 1200–1209. doi: 10.2741/2138
|
| [42] |
Videla LA. Cytoprotective and suicidal signaling in oxidative stress[J]. Biol Res, 2010, 43(3): 363–369.
|
| [43] |
Hines IN, Hoffman JM, Scheerens H, et al. Regulation of postischemic liver injury following different durations of ischemia[J]. Am J Physiol Gastrointest Liver Physiol, 2003, 284(3): G536–G545. doi: 10.1152/ajpgi.00400.2002
|
| [44] |
Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions[J]. Am J Physiol Gastrointest Liver Physiol, 2006, 290(6): G1083–G1088. doi: 10.1152/ajpgi.00568.2005
|
| [45] |
Spencer NY, Zhou WH, Li Q, et al. Hepatocytes produce TNF-α following hypoxia-reoxygenation and liver ischemia-reperfusion in a NADPH oxidase- and c-Src-dependent manner[J]. Am J Physiol Gastrointest Liver Physiol, 2013, 305(1): G84–G94. doi: 10.1152/ajpgi.00430.2012
|
| [46] |
Reiniers MJ, van Golen RF, van Gulik TM, et al. Reactive oxygen and nitrogen species in steatotic hepatocytes: a molecular perspective on the pathophysiology of ischemia-reperfusion injury in the fatty liver[J]. Antioxid Redox Signal, 2014, 21(7): 1119–1142. doi: 10.1089/ars.2013.5486
|
| [47] |
Pardini RS. Toxicity of oxygen from naturally occurring redox-active pro-oxidants[J]. Arch Insect Biochem Physiol, 1995, 29(2): 101–118. doi: 10.1002/arch.940290203
|
| [48] |
Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts[J]. J Gastroenterol Hepatol, 2011, 26(S1): 173–179.
|
| [49] |
Guicciardi ME, Malhi H, Mott JL, et al. Apoptosis and necrosis in the liver[J]. Compr Physiol, 2013, 3(2): 977–1010.
|
| [50] |
Rauen U, Polzar B, Stephan H, et al. Cold-induced apoptosis in cultured hepatocytes and liver endothelial cells: mediation by reactive oxygen species[J]. FASEB J, 1999, 13(1): 155–168. doi: 10.1096/fasebj.13.1.155
|
| [51] |
Kawada N, Tran-Thi TA, Klein H, et al. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances: Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus[J]. Eur J Biochem, 1993, 213(2): 815–823. doi: 10.1111/ejb.1993.213.issue-2
|
| [52] |
Kawamura E, Yamanaka N, Okamoto E, et al. Response of plasma and tissue endothelin-1 to liver ischemia and its implication in ischemia-reperfusion injury[J]. Hepatology, 1995, 21(4): 1138–1143. doi: 10.1016/0270-9139(95)90266-X
|
| [53] |
Lefer AM, Lefer DJ. Nitric oxide. II. Nitric oxide protects in intestinal inflammation[J]. Am J Physiol, 1999, 276(3 Pt 1): G572–G575.
|
| [54] |
Hamada T, Duarte S, Tsuchihashi S, et al. Inducible nitric oxide synthase deficiency impairs matrix metalloproteinase-9 activity and disrupts leukocyte migration in hepatic ischemia/reperfusion injury[J]. Am J Pathol, 2009, 174(6): 2265–2277. doi: 10.2353/ajpath.2009.080872
|
| [55] |
Abu-Amara M, Yang SY, Seifalian A, et al. The nitric oxide pathway-evidence and mechanisms for protection against liver ischaemia reperfusion injury[J]. Liver Int, 2012, 32(4): 531–543. doi: 10.1111/liv.2012.32.issue-4
|
| [56] |
Chen C, Lee WH, Zhong LW, et al. Regulatory T cells can mediate their function through the stimulation of APCs to produce immunosuppressive nitric oxide[J]. J Immunol, 2006, 176(6): 3449–3460. doi: 10.4049/jimmunol.176.6.3449
|
| [57] |
Phillips L, Toledo AH, Lopez-Neblina F, et al. Nitric oxide mechanism of protection in ischemia and reperfusion injury[J]. J Invest Surg, 2009, 22(1): 46–55. doi: 10.1080/08941930802709470
|
| [58] |
Lang JD Jr, Teng XJ, Chumley P, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation[J]. J Clin Invest, 2007, 117(9): 2583–2591. doi: 10.1172/JCI31892
|
| [59] |
Duranski MR, Greer JJM, Dejam A, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver[J]. J Clin Invest, 2005, 115(5): 1232–1240. doi: 10.1172/JCI22493
|
| [60] |
Li W, Meng ZH, Liu YL, et al. The hepatoprotective effect of sodium nitrite on cold ischemia-reperfusion injury[J]. J Transplant, 2012, 2012: 635179.
|
| [61] |
Shiratori Y, Kiriyama H, Fukushi Y, et al. Modulation of ischemia-reperfusion-induced hepatic injury by Kupffer cells[J]. Dig Dis Sci, 1994, 39(6): 1265–1272. doi: 10.1007/BF02093792
|
| [62] |
Jaeschke H, Bautista AP, Spolarics Z, et al. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats[J]. J Leukoc Biol, 1992, 52(4): 377–382. doi: 10.1002/jlb.1992.52.issue-4
|
| [63] |
Fondevila C, Shen XD, Tsuchihashi S, et al. The membrane attack complex (C5b-9) in liver cold ischemia and reperfusion injury[J]. Liver Transpl, 2008, 14(8): 1133–1141. doi: 10.1002/lt.v14:8
|
| [64] |
Brock RW, Nie RG, Harris KA, et al. Kupffer cell-initiated remote hepatic injury following bilateral hindlimb ischemia is complement dependent[J]. Am J Physiol Gastrointest Liver Physiol, 2001, 280(2): G279–G284. doi: 10.1152/ajpgi.2001.280.2.G279
|
| [65] |
Llacuna L, Marí M, Lluis JM, et al. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-κB inactivation in prolonged ischemia/reperfusion[J]. Am J Pathol, 2009, 174(5): 1776–1785. doi: 10.2353/ajpath.2009.080857
|
| [66] |
Selzner N, Selzner M, Odermatt B, et al. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-α/IL-6 in mice[J]. Gastroenterology, 2003, 124(3): 692–700. doi: 10.1053/gast.2003.50098
|
| [67] |
Boury NM, Czuprynski CJ. Listeria monocytogenes infection increases neutrophil adhesion and damage to a murine hepatocyte cell line in vitro[J]. Immunol Lett, 1995, 46(1–2): 111–116. doi: 10.1016/0165-2478(95)00027-3
|
| [68] |
Hanschen M, Zahler S, Krombach F, et al. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion[J]. Transplantation, 2008, 86(5): 710–718. doi: 10.1097/TP.0b013e3181821aa7
|
| [69] |
Nishimura Y, Takei Y, Kawano S, et al. The F(ab’)2 fragment of an anti-ICAM-1 monoclonal antibody attenuates liver injury after orthotopic liver transplantation[J]. Transplantation, 1996, 61(1): 99–104. doi: 10.1097/00007890-199601150-00020
|
| [70] |
Fong Y, Moldawer LL, Shires GT, et al. The biologic characteristics of cytokines and their implication in surgical injury[J]. Surg Gynecol Obstet, 1990, 170(4): 363–378.
|
| [71] |
Leifeld L, Cheng S, Ramakers J, et al. Imbalanced intrahepatic expression of interleukin 12, interferon gamma, and interleukin 10 in fulminant hepatitis B[J]. Hepatology, 2002, 36(4 Pt 1): 1001–1008.
|
| [72] |
Lentsch AB, Yoshidome H, Kato A, et al. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice[J]. Hepatology, 1999, 30(6): 1448–1453. doi: 10.1002/hep.510300615
|
| [73] |
Husted TL, Blanchard J, Schuster R, et al. Potential role for IL-23 in hepatic ischemia/reperfusion injury[J]. Inflamm Res, 2006, 55(5): 177–178. doi: 10.1007/s00011-006-0073-1
|
| [74] |
Colletti LM, Remick DG, Burtch GD, et al. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat[J]. J Clin Invest, 1990, 85(6): 1936–1943. doi: 10.1172/JCI114656
|
| [75] |
Colletti LM, Kunkel SL, Walz A, et al. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein[J]. J Clin Invest, 1995, 95(1): 134–141. doi: 10.1172/JCI117630
|
| [76] |
Colletti LM, Cortis A, Lukacs N, et al. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat[J]. Shock, 1998, 10(3): 182–191. doi: 10.1097/00024382-199809000-00006
|
| [77] |
Shito M, Wakabayashi G, Ueda M, et al. Interleukin 1 receptor blockade reduces tumor necrosis factor production, tissue injury, and mortality after hepatic ischemia-reperfusion in the rat[J]. Transplantation, 1997, 63(1): 143–148. doi: 10.1097/00007890-199701150-00026
|
| [78] |
Djeu JY, Matsushima K, Oppenheim JJ, et al. Functional activation of human neutrophils by recombinant monocyte-derived neutrophil chemotactic factor/IL-8[J]. J Immunol, 1990, 144(6): 2205–2210.
|
| [79] |
Lentsch AB, Yoshidome H, Cheadle WG, et al. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and Kupffer cells[J]. Hepatology, 1998, 27(2): 507–512. doi: 10.1002/hep.510270226
|
| [80] |
Ke BB, Shen XD, Lassman CR, et al. Cytoprotective and antiapoptotic effects of IL-13 in hepatic cold ischemia/reperfusion injury are heme oxygenase-1 dependent[J]. Am J Transplant, 2003, 3(9): 1076–1082. doi: 10.1034/j.1600-6143.2003.00147.x
|
| [81] |
Reiter RJ, Paredes SD, Manchester LC, et al. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin[J]. Crit Rev Biochem Mol Biol, 2009, 44(4): 175–200. doi: 10.1080/10409230903044914
|
| [82] |
López-Burillo S, Tan DX, Rodriguez-Gallego V, et al. Melatonin and its derivatives cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine and 6-methoxymelatonin reduce oxidative DNA damage induced by Fenton reagents[J]. J Pineal Res, 2003, 34(3): 178–184. doi: 10.1111/jpi.2003.34.issue-3
|
| [83] |
Barlow-Walden LR, Reiter RJ, Abe M, et al. Melatonin stimulates brain glutathione peroxidase activity[J]. Neurochem Int, 1995, 26(5): 497–502. doi: 10.1016/0197-0186(94)00154-M
|
| [84] |
Reiter RJ, Tan DX, Osuna C, et al. Actions of melatonin in the reduction of oxidative stress: a review[J]. J Biomed Sci, 2000, 7(6): 444–458. doi: 10.1007/BF02253360
|
| [85] |
Okatani Y, Wakatsuki A, Reiter RJ, et al. Protective effect of melatonin against mitochondrial injury induced by ischemia and reperfusion of rat liver[J]. Eur J Pharmacol, 2003, 469(1–3): 145–152. doi: 10.1016/S0014-2999(03)01643-1
|
| [86] |
Kireev R, Bitoun S, Cuesta S, et al. Melatonin treatment protects liver of Zucker rats after ischemia/reperfusion by diminishing oxidative stress and apoptosis[J]. Eur J Pharmacol, 2013, 701(1–3): 185–193. doi: 10.1016/j.ejphar.2012.11.038
|
| [87] |
Vairetti M, Ferrigno A, Bertone R, et al. Exogenous melatonin enhances bile flow and ATP levels after cold storage and reperfusion in rat liver: implications for liver transplantation[J]. J Pineal Res, 2005, 38(4): 223–230. doi: 10.1111/jpi.2005.38.issue-4
|
| [88] |
De Deken J, Rex S, Monbaliu D, et al. The efficacy of noble gases in the attenuation of ischemia reperfusion injury: a systematic review and meta-analyses[J]. Crit Care Med, 2016, 44(9): e886–e896. doi: 10.1097/CCM.0000000000001717
|
| [89] |
Wilke HJ, Moench C, Lotz G, et al. Xenon anesthesia for liver transplant surgery: a report of four cases[J]. Transplant Proc, 2011, 43(7): 2683–2686. doi: 10.1016/j.transproceed.2011.06.029
|
| [90] |
Thies JC, Teklote J, Clauer U, et al. The efficacy of N-acetylcysteine as a hepatoprotective agent in liver transplantation[J]. Transpl Int, 1998, 11(S1): S390–S392. doi: 10.1111/j.1432-2277.1998.tb01164.x
|
| [91] |
Weigand MA, Plachky J, Thies JC, et al. N-acetylcysteine attenuates the increase in α-glutathione S-transferase and circulating ICAM-1 and VCAM-1 after reperfusion in humans undergoing liver transplantation[J]. Transplantation, 2001, 72(4): 694–698. doi: 10.1097/00007890-200108270-00023
|
| [92] |
Bucuvalas JC, Ryckman FC, Krug S, et al. Effect of treatment with prostaglandin E1 and N-acetylcysteine on pediatric liver transplant recipients: a single-center study[J]. Pediatr Transplant, 2001, 5(4): 274–278. doi: 10.1034/j.1399-3046.2001.005004274.x
|
| [93] |
Bromley PN, Cottam SJ, Hilmi I, et al. Effects of intraoperative N-acetylcysteine in orthotopic liver transplantation[J]. Br J Anaesth, 1995, 75(3): 352–354. doi: 10.1093/bja/75.3.352
|
| [94] |
Steib A, Freys G, Collin F, et al. Does N-acetylcysteine improve hemodynamics and graft function in liver transplantation?[J]. Liver Transpl Surg, 1998, 4(2): 152–157. doi: 10.1002/(ISSN)1527-6473a
|
| [95] |
Tsuchihashi SI, Fondevila C, Shaw GD, et al. Molecular characterization of rat leukocyte P-selectin glycoprotein ligand-1 and effect of its blockade: protection from ischemia-reperfusion injury in liver transplantation[J]. J Immunol, 2006, 176(1): 616–624. doi: 10.4049/jimmunol.176.1.616
|
| [96] |
Dulkanchainun TS, Goss JA, Imagawa DK, et al. Reduction of hepatic ischemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1[J]. Ann Surg, 1998, 227(6): 832–840. doi: 10.1097/00000658-199806000-00006
|
| [97] |
Amersi F, Farmer DG, Shaw GD, et al. P-selectin glycoprotein ligand-1 (rPSGL-Ig)-mediated blockade of CD62 selectin molecules protects rat steatotic liver grafts from ischemia/reperfusion injury[J]. Am J Transplant, 2002, 2(7): 600–608. doi: 10.1034/j.1600-6143.2002.20704.x
|
| [98] |
Busuttil RW, Lipshutz GS, Kupiec-Weglinski JW, et al. rPSGL-Ig for improvement of early liver allograft function: a double-blind, placebo-controlled, single-center phase II study[J]. Am J Transplant, 2011, 11(4): 786–797. doi: 10.1111/j.1600-6143.2011.03441.x
|
| [99] |
Valentino KL, Gutierrez M, Sanchez R, et al. First clinical trial of a novel caspase inhibitor: anti-apoptotic caspase inhibitor, IDN-6556, improves liver enzymes[J]. Int J Clin Pharmacol Ther, 2003, 41(10): 441–449. doi: 10.5414/CPP41441
|
| [100] |
Linton SD, Aja T, Armstrong RA, et al. First-in-class pan caspase inhibitor developed for the treatment of liver disease[J]. J Med Chem, 2005, 48(22): 6779–6782. doi: 10.1021/jm050307e
|
| [101] |
Baskin-Bey ES, Washburn K, Feng S, et al. Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury[J]. Am J Transplant, 2007, 7(1): 218–225. doi: 10.1111/ajt.2007.7.issue-1
|
| [102] |
Song G, Ouyang GL, Bao SD. The activation of Akt/PKB signaling pathway and cell survival[J]. J Cell Mol Med, 2005, 9(1): 59–71. doi: 10.1111/jcmm.2005.9.issue-1
|
| [103] |
Covington SM, Bauler LD, Toledo-Pereyra LH. Akt: a therapeutic target in hepatic ischemia-reperfusion injury[J]. J Invest Surg, 2017, 30(1): 47–55. doi: 10.1080/08941939.2016.1206999
|
| [104] |
Koh PO. Melatonin prevents hepatic injury-induced decrease in Akt downstream targets phosphorylations[J]. J Pineal Res, 2011, 51(2): 214–219. doi: 10.1111/j.1600-079X.2011.00879.x
|
| [105] |
Bertoldo MJ, Faure M, Dupont J, et al. AMPK: a master energy regulator for gonadal function[J]. Front Neurosci, 2015, 9: 235.
|
| [106] |
Peralta C, Bartrons R, Serafin A, et al. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat[J]. Hepatology, 2001, 34(6): 1164–1173. doi: 10.1053/jhep.2001.29197
|
| [107] |
Ding WX, Zhang Q, Dong YB, et al. Adiponectin protects the rats liver against chronic intermittent hypoxia induced injury through AMP-activated protein kinase pathway[J]. Sci Rep, 2016, 6: 34151. doi: 10.1038/srep34151
|
| [108] |
Zhang CZ, Liao Y, Li Q, et al. Recombinant adiponectin ameliorates liver ischemia reperfusion injury via activating the AMPK/eNOS pathway[J]. PLoS One, 2013, 8(6): e66382. doi: 10.1371/journal.pone.0066382
|
| [109] |
Lehrke M, Lazar MA. The many faces of PPARγ[J]. Cell, 2005, 123(6): 993–999. doi: 10.1016/j.cell.2005.11.026
|
| [110] |
Marion-Letellier R, Savoye G, Ghosh S. Fatty acids, eicosanoids and PPAR gamma[J]. Eur J Pharmacol, 2016, 785: 44–49. doi: 10.1016/j.ejphar.2015.11.004
|
| [111] |
Zhou YL, Jia S, Wang CJ, et al. FAM3A is a target gene of peroxisome proliferator-activated receptor gamma[J]. Biochim Biophys Acta, 2013, 1830(8): 4160–4170. doi: 10.1016/j.bbagen.2013.03.029
|
| [112] |
Yang WL, Chen J, Meng YH, et al. Novel targets for treating ischemia-reperfusion injury in the liver[J]. Int J Mol Sci, 2018, 19(5): E1302. doi: 10.3390/ijms19051302
|
| [113] |
Xu CF, Yu CH, Li YM. Regulation of hepatic microRNA expression in response to ischemic preconditioning following ischemia/reperfusion injury in mice[J]. OMICS, 2009, 13(6): 513–520. doi: 10.1089/omi.2009.0035
|
| [114] |
Gehrau RC, Mas VR, Dumur CI, et al. Regulation of molecular pathways in ischemia-reperfusion injury after liver transplantation[J]. Transplantation, 2013, 96(10): 926–934. doi: 10.1097/TP.0b013e3182a20398
|
| [115] |
Mard SA, Akbari G, Dianat M, et al. Protective effects of crocin and zinc sulfate on hepatic ischemia-reperfusion injury in rats: a comparative experimental model study[J]. Biomed Pharmacother, 2017, 96: 48–55. doi: 10.1016/j.biopha.2017.09.123
|
| [116] |
Peralta C, Hotter G, Closa D, et al. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine[J]. Hepatology, 1997, 25(4): 934–937. doi: 10.1002/hep.510250424
|
| [117] |
Quarrie R, Cramer BM, Lee DS, et al. Ischemic preconditioning decreases mitochondrial proton leak and reactive oxygen species production in the postischemic heart[J]. J Surg Res, 2011, 165(1): 5–14. doi: 10.1016/j.jss.2010.09.018
|
| [118] |
Richards JA, Wigmore SJ, Devey LR. Heme oxygenase system in hepatic ischemia-reperfusion injury[J]. World J Gastroenterol, 2010, 16(48): 6068–6078. doi: 10.3748/wjg.v16.i48.6068
|
| [119] |
Liu AD, Fang HS, Wei WW, et al. Ischemic preconditioning protects against liver ischemia/reperfusion injury via heme oxygenase-1-mediated autophagy[J]. Crit Care Med, 2014, 42(12): e762–e771. doi: 10.1097/CCM.0000000000000659
|
| [120] |
Rüdiger HA, Graf R, Clavien PA. Sub-lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver[J]. J Hepatol, 2003, 39(6): 972–977. doi: 10.1016/S0168-8278(03)00415-X
|
| [121] |
Rolo AP, Teodoro JS, Peralta C, et al. Prevention of I/R injury in fatty livers by ischemic preconditioning is associated with increased mitochondrial tolerance: the key role of ATPsynthase and mitochondrial permeability transition[J]. Transpl Int, 2009, 22(11): 1081–1090. doi: 10.1111/tri.2009.22.issue-11
|
| [122] |
Abu-Amara M, Yang SY, Quaglia A, et al. Role of endothelial nitric oxide synthase in remote ischemic preconditioning of the mouse liver[J]. Liver Transpl, 2011, 17(5): 610–619. doi: 10.1002/lt.v17.5
|
| [123] |
Koti RS, Seifalian AM, Davidson BR. Protection of the liver by ischemic preconditioning: a review of mechanisms and clinical applications[J]. Dig Surg, 2003, 20(5): 383–396. doi: 10.1159/000072064
|
| [124] |
Gurusamy KS, Kumar Y, Sharma D, et al. Ischaemic preconditioning for liver transplantation[J]. Cochrane Database Syst Rev, 2008, (1): CD006315.
|
| [125] |
Nadarajah L, Yaqoob MM, McCafferty K. Ischemic conditioning in solid organ transplantation: is it worth giving your right arm for?[J]. Curr Opin Nephrol Hypertens, 2017, 26(6): 467–476. doi: 10.1097/MNH.0000000000000367
|
| [126] |
Koneru B, Fisher A, He Y, et al. Ischemic preconditioning in deceased donor liver transplantation: a prospective randomized clinical trial of safety and efficacy[J]. Liver Transpl, 2005, 11(2): 196–202. doi: 10.1002/(ISSN)1527-6473
|
| [127] |
Koneru B, Shareef A, Dikdan G, et al. The ischemic preconditioning paradox in deceased donor liver transplantation-evidence from a prospective randomized single blind clinical trial[J]. Am J Transplant, 2007, 7(12): 2788–2796. doi: 10.1111/ajt.2007.7.issue-12
|
| [128] |
Theodoraki K, Karmaniolou I, Tympa A, et al. Beyond preconditioning: postconditioning as an alternative technique in the prevention of liver ischemia-reperfusion injury[J]. Oxid Med Cell Longev, 2016, 2016: 8235921.
|
| [129] |
Sun K, Liu ZS, Sun Q. Role of mitochondria in cell apoptosis during hepatic ischemia-reperfusion injury and protective effect of ischemic postconditioning[J]. World J Gastroenterol, 2004, 10(13): 1934–1938. doi: 10.3748/wjg.v10.i13.1934
|
| [130] |
Zhang WX, Yin W, Zhang L, et al. Preconditioning and postconditioning reduce hepatic ischemia-reperfusion injury in rats[J]. Hepatobiliary Pancreat Dis Int, 2009, 8(6): 586–590.
|
| [131] |
Yoon SY, Kim CY, Han HJ, et al. Protective effect of ischemic postconditioning against hepatic ischemic reperfusion injury in rat liver[J]. Ann Surg Treat Res, 2015, 88(5): 241–245. doi: 10.4174/astr.2015.88.5.241
|
| [132] |
Lin HC, Lee TK, Tsai CC, et al. Ischemic postconditioning protects liver from ischemia-reperfusion injury by modulating mitochondrial permeability transition[J]. Transplantation, 2012, 93(3): 265–271. doi: 10.1097/TP.0b013e31823ef335
|
| [133] |
Wang N, Lu JG, He XL, et al. Effects of ischemic postconditioning on reperfusion injury in rat liver grafts after orthotopic liver transplantation[J]. Hepatol Res, 2009, 39(4): 382–390. doi: 10.1111/hep.2009.39.issue-4
|
| [134] |
Kim WH, Lee JH, Ko JS, et al. Effect of remote ischemic postconditioning on patients undergoing living donor liver transplantation[J]. Liver Transpl, 2014, 20(11): 1383–1392. doi: 10.1002/lt.23960
|
| [135] |
Ricca L, Lemoine A, Cauchy F, et al. Ischemic postconditioning of the liver graft in adult liver transplantation[J]. Transplantation, 2015, 99(8): 1633–1643. doi: 10.1097/TP.0000000000000685
|
| [136] |
Schlegel AA, Kalisvaart M, Muiesan P. Machine perfusion in liver transplantation: an essential treatment or just an expensive toy?[J]. Minerva Anestesiol, 2018, 84(2): 236–245.
|
| [137] |
Liu Q, Vekemans K, Iania L, et al. Assessing warm ischemic injury of pig livers at hypothermic machine perfusion[J]. J Surg Res, 2014, 186(1): 379–389. doi: 10.1016/j.jss.2013.07.034
|
| [138] |
Monbaliu D, Liu Q, Libbrecht L, et al. Preserving the morphology and evaluating the quality of liver grafts by hypothermic machine perfusion: a proof-of-concept study using discarded human livers[J]. Liver Transpl, 2012, 18(12): 1495–1507. doi: 10.1002/lt.v18.12
|
| [139] |
Manekeller S, Schuppius A, Stegemann J, et al. Role of perfusion medium, oxygen and rheology for endoplasmic reticulum stress-induced cell death after hypothermic machine preservation of the liver[J]. Transpl Int, 2008, 21(2): 169–177.
|
| [140] |
Jain S, Xu HZ, Duncan H, et al. Ex-vivo study of flow dynamics and endothelial cell structure during extended hypothermic machine perfusion preservation of livers[J]. Cryobiology, 2004, 48(3): 322–332. doi: 10.1016/j.cryobiol.2004.01.010
|
| [141] |
Schlegel A, de Rougemont O, Graf R, et al. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts[J]. J Hepatol, 2013, 58(2): 278–286. doi: 10.1016/j.jhep.2012.10.004
|
| [142] |
Gallinat A, Efferz P, Paul A, et al. One or 4 h of " in-house” reconditioning by machine perfusion after cold storage improve reperfusion parameters in porcine kidneys[J]. Transpl Int, 2014, 27(11): 1214–1219. doi: 10.1111/tri.2014.27.issue-11
|
| [143] |
Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation facilitates successful transplantation of " orphan” extended criteria donor livers[J]. Am J Transplant, 2015, 15(1): 161–169. doi: 10.1111/ajt.12958
|
| [144] |
Dutkowski P, Schlegel A, de Oliveira M, et al. HOPE for human liver grafts obtained from donors after cardiac death[J]. J Hepatol, 2014, 60(4): 765–772. doi: 10.1016/j.jhep.2013.11.023
|
| [145] |
Schlegel A, Muller X, Kalisvaart M, et al. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation[J]. J Hepatol, 2019, 70(1): 50–57.
|
| [146] |
Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial[J]. Am J Transplant, 2016, 16(6): 1779–1787. doi: 10.1111/ajt.13708
|
| [147] |
Xu HZ, Berendsen T, Kim K, et al. Excorporeal normothermic machine perfusion resuscitates pig DCD livers with extended warm ischemia[J]. J Surg Res, 2012, 173(2): e83–e88. doi: 10.1016/j.jss.2011.09.057
|
| [148] |
Mergental H, Perera MTPR, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation[J]. Am J Transplant, 2016, 16(11): 3235–3245. doi: 10.1111/ajt.13875
|
| [149] |
Jassem W, Xystrakis E, Ghnewa YG, et al. Normothermic machine perfusion (NMP) inhibits proinflammatory responses in the liver and promotes regeneration[J]. Hepatology, 2018. doi: 10.1002/hep.30475[Epub ahead of print
|
| [150] |
Balfoussia D, Yerrakalva D, Hamaoui K, et al. Advances in machine perfusion graft viability assessment in kidney, liver, pancreas, lung, and heart transplant[J]. Exp Clin Transplant, 2012, 10(2): 87–100. doi: 10.6002/ect
|
| [151] |
Watson CJE, Randle LV, Kosmoliaptsis V, et al. 26-hour storage of a declined liver before successful transplantation using ex vivo normothermic perfusion[J]. Ann Surg, 2017, 265(1): e1–e2. doi: 10.1097/SLA.0000000000001834
|
| [152] |
Laing RW, Bhogal RH, Wallace L, et al. The use of an acellular oxygen carrier in a human liver model of normothermic machine perfusion[J]. Transplantation, 2017, 101(11): 2746–2756. doi: 10.1097/TP.0000000000001821
|
| [153] |
op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers[J]. Am J Transplant, 2013, 13(5): 1327–1335. doi: 10.1111/ajt.12187
|
| [154] |
Braat AE, Blok JJ, Putter H, et al. The eurotransplant donor risk index in liver transplantation: ET-DRI[J]. Am J Transplant, 2012, 12(10): 2789–2796. doi: 10.1111/j.1600-6143.2012.04195.x
|
| [155] |
Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index[J]. Am J Transplant, 2006, 6(4): 783–790. doi: 10.1111/j.1600-6143.2006.01242.x
|
| [156] |
Perera T, Mergental H, Stephenson B, et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion[J]. Liver Transpl, 2016, 22(1): 120–124. doi: 10.1002/lt.24369
|
| [157] |
Watson CJE, Kosmoliaptsis V, Randle LV, et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia-important lessons from the first 12 cases[J]. Transplantation, 2017, 101(5): 1084–1098. doi: 10.1097/TP.0000000000001661
|
| [158] |
Khorsandi SE, Quaglia A, Salehi S, et al. The microRNA expression profile in donation after cardiac death (DCD) livers and its ability to identify primary non function[J]. PLoS One, 2015, 10(5): e0127073. doi: 10.1371/journal.pone.0127073
|
| [159] |
Bruinsma BG, Sridharan GV, Weeder PD, et al. Metabolic profiling during ex vivo machine perfusion of the human liver[J]. Sci Rep, 2016, 6: 22415. doi: 10.1038/srep22415
|
| [160] |
Nasralla D, Coussios CC, Mergental H, et al. A randomized trial of normothermic preservation in liver transplantation[J]. Nature, 2018, 557(7703): 50–56. doi: 10.1038/s41586-018-0047-9
|
| [161] |
Durand F, Renz JF, Alkofer B, et al. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation[J]. Liver Transpl, 2008, 14(12): 1694–1707. doi: 10.1002/lt.v14:12
|
| [162] |
Spitzer AL, Lao OB, Dick AAS, et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment[J]. Liver Transpl, 2010, 16(7): 874–884. doi: 10.1002/lt.v16:7
|
| [163] |
Nativ NI, Maguire TJ, Yarmush G, et al. Liver defatting: an alternative approach to enable steatotic liver transplantation[J]. Am J Transplant, 2012, 12(12): 3176–3183. doi: 10.1111/ajt.2012.12.issue-12
|
| [164] |
Nagrath D, Xu HZ, Tanimura Y, et al. Metabolic preconditioning of donor organs: defatting fatty livers by normothermic perfusion ex vivo[J]. Metab Eng, 2009, 11(4–5): 274–283. doi: 10.1016/j.ymben.2009.05.005
|
| [165] |
Boteon YL, Afford SC, Mergental H. Pushing the limits: machine preservation of the liver as a tool to recondition high-risk grafts[J]. Curr Transplant Rep, 2018, 5(2): 113–120. doi: 10.1007/s40472-018-0188-7
|
| [166] |
Goldaracena N, Echeverri J, Spetzler VN, et al. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation[J]. Liver Transpl, 2016, 22(11): 1573–1583. doi: 10.1002/lt.v22.11
|
| [167] |
Morales-Ruiz M, Fondevila C, Muñoz-Luque J, et al. Gene transduction of an active mutant of akt exerts cytoprotection and reduces graft injury after liver transplantation[J]. Am J Transplant, 2007, 7(4): 769–778. doi: 10.1111/ajt.2007.7.issue-4
|
| [168] |
Van Raemdonck D, Neyrinck A, Rega F, et al. Machine perfusion in organ transplantation: a tool for ex-vivo graft conditioning with mesenchymal stem cells?[J]. Curr Opin Organ Transplant, 2013, 18(1): 24–33. doi: 10.1097/MOT.0b013e32835c494f
|
| [1] | Tiwari-Heckler Shilpa, Jiang Z. Gordon, Popov Yury, J. Mukamal Kenneth. Daily high-dose aspirin does not lower APRI in the Aspirin-Myocardial Infarction Study[J]. The Journal of Biomedical Research, 2020, 34(2): 139-142. DOI: 10.7555/JBR.33.20190041 |
| [2] | Tao Chun'ai, Gan Yongxin, Su Weidong, Li Zhutian, Tang Xiaolan. Effectiveness of hospital disinfection and experience learnt from 11 years of surveillance[J]. The Journal of Biomedical Research, 2019, 33(6): 408-413. DOI: 10.7555/JBR.33.20180118 |
| [3] | Huan Liu, Shijiang Zhang, Yongfeng Shao, Xiaohu Lu, Weidong Gu, Buqing Ni, Qun Gu, Junjie Du. Biomechanical characterization of a novel ring connector for sutureless aortic anastomosis[J]. The Journal of Biomedical Research, 2018, 32(6): 454-460. DOI: 10.7555/JBR.31.20170011 |
| [4] | Minbo Zang, Qiao Zhou, Yunfei Zhu, Mingxi Liu, Zuomin Zhou. Effects of chemotherapeutic agent bendamustine for nonhodgkin lymphoma on spermatogenesis in mice[J]. The Journal of Biomedical Research, 2018, 32(6): 442-453. DOI: 10.7555/JBR.31.20170023 |
| [5] | Kaibo Lin, Shikun Zhang, Jieli Chen, Ding Yang, Mengyi Zhu, Eugene Yujun Xu. Generation and functional characterization of a conditional Pumilio2 null allele[J]. The Journal of Biomedical Research, 2018, 32(6): 434-441. DOI: 10.7555/JBR.32.20170117 |
| [6] | Huanqiang Wang, Congying Yang, Siyuan Wang, Tian Wang, Jingling Han, Kai Wei, Fucun Liu, Jida Xu, Xianzhen Peng, Jianming Wang. Cell-free plasma hypermethylated CASZ1, CDH13 and ING2 are promising biomarkers of esophageal cancer[J]. The Journal of Biomedical Research, 2018, 32(6): 424-433. DOI: 10.7555/JBR.32.20170065 |
| [7] | Fengzhen Wang, Mingwan Zhang, Dongsheng Zhang, Yuan Huang, Li Chen, Sunmin Jiang, Kun Shi, Rui Li. Preparation, optimization, and characterization of chitosancoated solid lipid nanoparticles for ocular drug delivery[J]. The Journal of Biomedical Research, 2018, 32(6): 411-423. DOI: 10.7555/JBR.32.20160170 |
| [8] | Christopher J. Danford, Zemin Yao, Z. Gordon Jiang. Non-alcoholic fatty liver disease: a narrative review of genetics[J]. The Journal of Biomedical Research, 2018, 32(6): 389-400. DOI: 10.7555/JBR.32.20180045 |
| [9] | Nolan B. Ayers, Chenming Sun, Shi-You Chen. Transforming growth factor-β signaling in systemic sclerosis[J]. The Journal of Biomedical Research, 2018, 32(1): 3-12. DOI: 10.7555/JBR.31.20170034 |
| [10] | Ashish Kumar Sharma, Arshee Munajjam, Bhawna Vaishnav, Richa Sharma, Ashok Sharma, Kunal Kishore, Akash Sharma, Divya Sharma, Rita Kumari, Ashish Tiwari, Santosh Kumar Singh, Samir Gaur, Vijay Singh Jatav, Barthu Parthi Srinivasan, Shyam Sunder Agarwal. Involvement of adenosine and standardization of aqueous extract of garlic (Allium sativum Linn.) on cardioprotective and cardiodepressant properties in ischemic preconditioning and myocardial ischemia-reperfusion induced cardiac injury[J]. The Journal of Biomedical Research, 2012, 26(1): 24-36. DOI: 10.1016/S1674-8301(12)60004-9 |
| 1. | Guo L, Yang Q, Zhu J, et al. REGγ deficiency ameliorates hepatic ischemia and reperfusion injury in a mitochondrial p66shc dependent manner in mice. Transl Gastroenterol Hepatol, 2024, 9: 62. DOI:10.21037/tgh-24-46 |
| 2. | Garzali IU, Aloun A, Abuzeid EED, et al. Early outcome of machine perfusion vs static cold storage of liver graft: A systemic review and meta-analysis of randomized controlled trials. Hepatol Forum, 2024, 5(4): 211-216. DOI:10.14744/hf.2023.2023.0069 |
| 3. | Puspita R, Jusuf AA, Antarianto RD, et al. A systematic review of the anti-inflammatory and anti-fibrotic potential of human umbilical cord mesenchymal stem cells-derived exosomes in experimental models of liver regeneration. Mol Biol Rep, 2024, 51(1): 999. DOI:10.1007/s11033-024-09929-0 |
| 4. | Xiong Y, Chen J, Liang W, et al. Blockade of the mitochondrial DNA release ameliorates hepatic ischemia-reperfusion injury through avoiding the activation of cGAS-Sting pathway. J Transl Med, 2024, 22(1): 796. DOI:10.1186/s12967-024-05588-8 |
| 5. | Cortes-Mejia NA, Bejarano-Ramirez DF, Guerra-Londono JJ, et al. Portal vein arterialization in 25 liver transplant recipients: A Latin American single-center experience. World J Transplant, 2024, 14(2): 92528. DOI:10.5500/wjt.v14.i2.92528 |
| 6. | Faleiro MD, Mir ZM, Azizieh Y, et al. Oncologic Outcomes of Interventions to Decrease Allograft Ischemia-Reperfusion Injury within Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma: A Systematic Review. Curr Oncol, 2024, 31(6): 2895-2906. DOI:10.3390/curroncol31060221 |
| 7. | Goossens C, Tambay V, Raymond VA, et al. Impact of the delay in cryopreservation timing during biobanking procedures on human liver tissue metabolomics. PLoS One, 2024, 19(6): e0304405. DOI:10.1371/journal.pone.0304405 |
| 8. | Platt E, Robertson F, Al-Rashed A, et al. NGAL in the Development of Acute Kidney Injury in a Murine Model of Remote Ischaemic Preconditioning and Liver Ischaemia Reperfusion. Int J Mol Sci, 2024, 25(10): 5061. DOI:10.3390/ijms25105061 |
| 9. | Ghasemi Pour Afshar N, Arab HA, Vatannejad A, et al. The Role of the JAK-STAT Signaling Pathway in the Protective Effects of Hepatic Ischemia Post-conditioning Against the Injury Induced by Ischemia/Reperfusion in the Rat Liver. Adv Pharm Bull, 2024, 14(1): 224-230. DOI:10.34172/apb.2024.003 |
| 10. | Wilson EA, Woodbury A, Williams KM, et al. OXIDATIVE study: A pilot prospective observational cohort study protocol examining the influence of peri-reperfusion hyperoxemia and immune dysregulation on early allograft dysfunction after orthotopic liver transplantation. PLoS One, 2024, 19(3): e0301281. DOI:10.1371/journal.pone.0301281 |
| 11. | Babboni S, Vacca PG, Simonini L, et al. Cholangiocyte Organoids: The New Frontier in Regenerative Medicine for the Study and Treatment of Cholangiopathies. J Clin Med, 2024, 13(6): 1804. DOI:10.3390/jcm13061804 |
| 12. | Zhang Y, Lv J, Bai J, et al. METTL3 Modulates TXNIP Expression to Affect the Activation of NLRP3 Inflammasome in Hepatic Cells Under Oxygen-Glucose Deprivation/Reperfusion Injury. Inflammation, 2024, 47(3): 1028-1040. DOI:10.1007/s10753-023-01958-4 |
| 13. | Vargas PA, Yu C, Goldaracena N. Comprehensive review of the application of MP and the potential for graft modification. Front Transplant, 2023, 2: 1163539. DOI:10.3389/frtra.2023.1163539 |
| 14. | Mouratidou C, Pavlidis ET, Katsanos G, et al. Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog. World J Gastrointest Surg, 2023, 15(9): 1858-1870. DOI:10.4240/wjgs.v15.i9.1858 |
| 15. | Eissa AM, Hassanin MH, Ibrahim IAAEH. Hepatic β-arrestins: potential roles in liver health and disease. Mol Biol Rep, 2023, 50(12): 10399-10407. DOI:10.1007/s11033-023-08898-0 |
| 16. | Khalil A, Quaglia A, Gélat P, et al. New Developments and Challenges in Liver Transplantation. J Clin Med, 2023, 12(17): 5586. DOI:10.3390/jcm12175586 |
| 17. | Kahan R, Cray PL, Abraham N, et al. Sterile inflammation in liver transplantation. Front Med (Lausanne), 2023, 10: 1223224. DOI:10.3389/fmed.2023.1223224 |
| 18. | Valdés S, Paredes SD, García Carreras C, et al. S-Adenosylmethionine Decreases Bacterial Translocation, Proinflammatory Cytokines, Oxidative Stress and Apoptosis Markers in Hepatic Ischemia-Reperfusion Injury in Wistar Rats. Antioxidants (Basel), 2023, 12(8): 1539. DOI:10.3390/antiox12081539 |
| 19. | Cooper SA, Kostallari E, Shah VH. Angiocrine Signaling in Sinusoidal Health and Disease. Semin Liver Dis, 2023, 43(3): 245-257. DOI:10.1055/a-2128-5907 |
| 20. | Sedik AA, Hassan A, Salama A. Synergistic effect of arginine and Lactobacillus plantarum against potassium dichromate induced-acute liver and kidney injury in rats: Role of iNOS and TLR4/NF-κB signaling pathways. Iran J Basic Med Sci, 2023, 26(8): 941-952. DOI:10.22038/IJBMS.2023.68855.15108 |
| 21. | Hofmann J, Meszaros AT, Buch ML, et al. Bioenergetic and Cytokine Profiling May Help to Rescue More DCD Livers for Transplantation. Int J Mol Sci, 2023, 24(11): 9536. DOI:10.3390/ijms24119536 |
| 22. | Azizieh Y, Westhaver LP, Badrudin D, et al. Changing liver utilization and discard rates in clinical transplantation in the ex-vivo machine preservation era. Front Med Technol, 2023, 5: 1079003. DOI:10.3389/fmedt.2023.1079003 |
| 23. | Roushansarai NS, Pascher A, Becker F. Innate Immune Cells during Machine Perfusion of Liver Grafts-The Janus Face of Hepatic Macrophages. J Clin Med, 2022, 11(22): 6669. DOI:10.3390/jcm11226669 |
| 24. | Sayaf K, Gabbia D, Russo FP, et al. The Role of Sex in Acute and Chronic Liver Damage. Int J Mol Sci, 2022, 23(18): 10654. DOI:10.3390/ijms231810654 |
| 25. | Peng Y, Yin Q, Yuan M, et al. Role of hepatic stellate cells in liver ischemia-reperfusion injury. Front Immunol, 2022, 13: 891868. DOI:10.3389/fimmu.2022.891868 |
| 26. | Zhang L, Cui LL, Yang WH, et al. Effect of intraoperative dexmedetomidine on hepatic ischemia-reperfusion injury in pediatric living-related liver transplantation: A propensity score matching analysis. Front Surg, 2022, 9: 939223. DOI:10.3389/fsurg.2022.939223 |
| 27. | Matsunaga T, Roesel MJ, Schroeter A, et al. Preserving and rejuvenating old organs for transplantation: novel treatments including the potential of senolytics. Curr Opin Organ Transplant, 2022, 27(5): 481-487. DOI:10.1097/MOT.0000000000001019 |
| 28. | Kaltenmeier C, Yazdani HO, Handu S, et al. The Role of Neutrophils as a Driver in Hepatic Ischemia-Reperfusion Injury and Cancer Growth. Front Immunol, 2022, 13: 887565. DOI:10.3389/fimmu.2022.887565 |
| 29. | Zhang X, Li J, Liu T, et al. Identification of Key Biomarkers and Immune Infiltration in Liver Tissue after Bariatric Surgery. Dis Markers, 2022, 2022: 4369329. DOI:10.1155/2022/4369329 |
| 30. | Zhang YP, Liu XR, Yang MW, et al. New progress in understanding roles of nitric oxide during hepatic ischemia-reperfusion injury. World J Hepatol, 2022, 14(3): 504-515. DOI:10.4254/wjh.v14.i3.504 |
| 31. | Ingram H, Dogan M, Eason JD, et al. MicroRNAs: Novel Targets in Hepatic Ischemia-Reperfusion Injury. Biomedicines, 2022, 10(4): 791. DOI:10.3390/biomedicines10040791 |
| 32. | An W, Kang JS. Effect of Metformin on Myocardial Injury Induced by Hepatic Ischemia-Reperfusion in Rats. Front Pharmacol, 2022, 13: 822743. DOI:10.3389/fphar.2022.822743 |
| 33. | Hallett JM, Ferreira-Gonzalez S, Man TY, et al. Human biliary epithelial cells from discarded donor livers rescue bile duct structure and function in a mouse model of biliary disease. Cell Stem Cell, 2022, 29(3): 355-371. DOI:10.1016/j.stem.2022.02.006 |
| 34. | Platt E, Klootwijk E, Salama A, et al. Literature review of the mechanisms of acute kidney injury secondary to acute liver injury. World J Nephrol, 2022, 11(1): 13-29. DOI:10.5527/wjn.v11.i1.13 |
| 35. | Zhang Y, Li Y, Wang Q, et al. Attenuation of hepatic ischemia‑reperfusion injury by adipose stem cell‑derived exosome treatment via ERK1/2 and GSK‑3β signaling pathways. Int J Mol Med, 2022, 49(2): 13. DOI:10.3892/ijmm.2021.5068 |
| 36. | Kim HY, Choi B, Kim M, et al. Reusing hepatic grafts in Korea: a case report. Korean J Transplant, 2021, 35(3): 200-206. DOI:10.4285/kjt.21.0005 |
| 37. | Krzystek-Korpacka M, Fleszar MG, Fortuna P, et al. Modulation of Prostanoids Profile and Counter-Regulation of SDF-1α/CXCR4 and VIP/VPAC2 Expression by Sitagliptin in Non-Diabetic Rat Model of Hepatic Ischemia-Reperfusion Injury. Int J Mol Sci, 2021, 22(23): 13155. DOI:10.3390/ijms222313155 |
| 38. | Herrera Vielma F, Valenzuela R, Videla LA, et al. N-3 Polyunsaturated Fatty Acids and Their Lipid Mediators as A Potential Immune-Nutritional Intervention: A Molecular and Clinical View in Hepatic Disease and Other Non-Communicable Illnesses. Nutrients, 2021, 13(10): 3384. DOI:10.3390/nu13103384 |
| 39. | Ren Y, Lin S, Liu W, et al. Hepatic Remote Ischemic Preconditioning (RIPC) Protects Heart Damages Induced by Ischemia Reperfusion Injury in Mice. Front Physiol, 2021, 12: 713564. DOI:10.3389/fphys.2021.713564 |
| 40. | Zhang L, Li N, Cui LL, et al. Intraoperative Low-Dose Dexmedetomidine Administration Associated with Reduced Hepatic Ischemia-Reperfusion Injury in Pediatric Deceased Liver Transplantation: A Retrospective Cohort Study. Ann Transplant, 2021, 26: e933354. DOI:10.12659/AOT.933354 |
| 41. | Felli E, Al-Taher M, Collins T, et al. Automatic Liver Viability Scoring with Deep Learning and Hyperspectral Imaging. Diagnostics (Basel), 2021, 11(9): 1527. DOI:10.3390/diagnostics11091527 |
| 42. | Ali ES, Rychkov GY, Barritt GJ. TRPM2 Non-Selective Cation Channels in Liver Injury Mediated by Reactive Oxygen Species. Antioxidants (Basel), 2021, 10(8): 1243. DOI:10.3390/antiox10081243 |
| 43. | Trocha M, Fleszar MG, Fortuna P, et al. Sitagliptin Modulates Oxidative, Nitrative and Halogenative Stress and Inflammatory Response in Rat Model of Hepatic Ischemia-Reperfusion. Antioxidants (Basel), 2021, 10(8): 1168. DOI:10.3390/antiox10081168 |
| 44. | López-López V, Pérez-Sánz F, de Torre-Minguela C, et al. Proteomics in Liver Transplantation: A Systematic Review. Front Immunol, 2021, 12: 672829. DOI:10.3389/fimmu.2021.672829 |
| 45. | Clarke G, Mergental H, Hann A, et al. How Machine Perfusion Ameliorates Hepatic Ischaemia Reperfusion Injury. Int J Mol Sci, 2021, 22(14): 7523. DOI:10.3390/ijms22147523 |
| 46. | Felli E, Muttillo EM, Felli E. Interpatient heterogeneity in hepatic microvascular blood flow during vascular inflow occlusion (Pringle manoeuvre). Hepatobiliary Surg Nutr, 2021, 10(3): 413-415. DOI:10.21037/hbsn-21-91 |
| 47. | Kulkarni A, Nadler JL, Mirmira RG, et al. Regulation of Tissue Inflammation by 12-Lipoxygenases. Biomolecules, 2021, 11(5): 717. DOI:10.3390/biom11050717 |
| 48. | Ferreira-Silva M, Faria-Silva C, Baptista PV, et al. Drug delivery nanosystems targeted to hepatic ischemia and reperfusion injury. Drug Deliv Transl Res, 2021, 11(2): 397-410. DOI:10.1007/s13346-021-00915-8 |
| 49. | Drescher S, van Hoogevest P. The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics, 2020, 12(12): 1235. DOI:10.3390/pharmaceutics12121235 |
| 50. | Ronca V, Wootton G, Milani C, et al. The Immunological Basis of Liver Allograft Rejection. Front Immunol, 2020, 11: 2155. DOI:10.3389/fimmu.2020.02155 |