| Citation: | Zhang Yuxin, Wang Shanshan, Xu Miao, Pang Jijing, Yuan Zhilan, Zhao Chen. AAV-mediated human CNGB3 restores cone function in an all-cone mouse model of CNGB3 achromatopsia[J]. The Journal of Biomedical Research, 2020, 34(2): 114-121. DOI: 10.7555/JBR.33.20190056 |
In the human retina, photoreceptor cyclic nucleotide-gated (CNG) channels play a pivotal role in phototransduction. Phototransduction relies on the function of CNG channels in both rod and cone photoreceptor outer segment plasma membranes[1– 2]. Structurally, CNG channels comprise two structurally related A and B subunits: the rod photoreceptor CNG channel is composed of CNGA1 and CNGB1 subunits, whereas the cone photoreceptor is composed of CNGA3 and CNGB3 subunits[2– 6]. Recent studies have shown that mutations in CNGA3 or CNGB3 cause clinically indistinguishable forms of congenital achromatopsia (ACHM)[7].
ACHM or rod monochromatism is an autosomal recessive hereditary visual disorder characterized by the absence of functional cone photoreceptors in infancy, affecting one in approximately 30 000 individuals worldwide[7]. Affected individuals exhibit pendular nystagmus, poor visual acuity, and photophobia[8]. The first signs of ACHM in infancy are nystagmus and photophobia as evidenced by squinting in bright light[8]. To date, mutations in six different genes have been identified as responsible for ACHM: CNGA3, CNGB3, GNAT2, PDE6C, PDE6H, and ATF6. Clinically, approximately 50% of ACHM patients are associated with CNGB3 mutations, about 25% with CNGA3 mutations and smaller fractions with mutations in the cone transducin or phosphodiesterase genes in European populations[7–12]. Previous studies demonstrated that the CNGA3 is the ion-conducting subunit, while the CNGB3 fails to form functional homomeric channels in heterologous expression systems when expresses alone[13– 14]. Because the B subunits are only thought to confer specific biophysical properties to the CNG channel complex whereas the A subunits contribute to the principal channel properties[9,15]. Therefore, the A subunits are thought to be the primary subunits while the B subunits play a modulatory role for the function of both rod and cone CNG channels[9,15]. Hence, knock-out of the gene encoding Cngb3 in mouse retina would cause a moderate reduction of the cone function[16].
Recent clinic trails on patients with Leber congenital amaurosis (LCA) demonstrated the successful expression of the RPE65 gene with adeno-associated viral (AAV) vectors[17–19], which provided a possible treatment for retinal degeneration including ACHM. Restoration of cone function and improvement in photopic vision were achieved in mouse and dog models of ACHM by subretinal (SR) delivery of AAV vectors[20– 21]. Progresses in AAV-based gene replacement therapy to restore cone-mediated function in Cngb3 deficient mouse models provided a foundation for the development of clinical trials for human ACHM patients[21– 22]. In these studies, AAV vectors containing human CNGB3 cDNA were delivered subretinally in Cngb3−/− mice, which carry a naturally occurring mutation in the Cngb3 gene.
We chose the Cngb3−/−/Nrl−/− mouse model because it possessed a genetically and phenotypically well-characterized cone degeneration. This double knock-out mouse had a retinal phenotype similar to its single knock-out Cngb3−/− mouse with impaired cone function and cone degeneration. Cngb3−/−/Nrl−/− mice lacked scotopic light response due to Nrl deficiency. The expression levels of cone arrestin and S-opsin were reduced in Cngb3−/−/Nrl−/− mice[16]. In addition, we speculate that this mouse model will respond well to SR gene-based therapy since rescue has been achieved in Cngb3−/− mice. Here, human CNGB3 expression was achieved using AAV8 (Y447, 733F) vector driven by a cone-specific promoter to test whether AAV8-(Y447, 733F)-PR2.1-hCNGB3 can rescue Cngb3−/−/Nrl−/− cones when delivered to the SR space.
C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, USA). Cngb3−/−[23] and Nrl−/−[24] mice were kindly provided by Dr. Anand Swaroop at NEI/NIH. Cngb3−/−/Nrl−/− mice were generated as described previously[16]. All animals were maintained under standard laboratory conditions (18 °C–23 °C, 40%–65% humidity) with food and water available ad libitum in the University of Florida Health Science Center Animal Care Service Facilities a 12-hour/12-hour light/dark cycle with <15 ft-c environmental illumination. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Florida and conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and National Institutes of Health regulations.
Human CNGB3 cDNA (purchased from American Type Culture Collection, USA) was cloned under the human PR2.1 promoter which has been shown to target transgene expression to mammalian L/M cones[25] to make AAV-PR2.1-hCNGB3 construct. The construct was packaged in AAV8 (Y447, 733F). The vectors were purified and tittered according to previously published methods[26].
At postnatal day 14 (P14), one microliter of AAV8 (Y447, 733F)-PR2.1-hCNGB3 vector (1013 vector genome/mL) was injected subretinally into one eye of each Cngb3−/−/Nrl−/− mouse. Transcorneal SR injections[27] were performed with a 33 gauge blunt needle mounted on a 5 mL Hamilton syringe. First, an entering hole was introduced at the edge of the corneal by a 30 gauge disposable needle, then the Hamilton syringe loaded with 1 µL of viral vector mixed with fluorescein dye was glided through the cornea opening into the subretinal space with previously described methods[28– 29]. The other eye remained untreated as a control. All eyes used for further evaluation had no apparent surgical complications and more than 80% of retinal detachment.
Electroretinography was performed at six months following SR injections. A UTAS Visual Diagnostic System with a Big Shot Ganzfeld (LKC Technologies, USA) was used for ERG recording. All mice were anesthetized by ketamine (72 mg/kg)/xylazine (4 mg/kg) by intraperitoneal injection. The pupils were dilated with 1% atropine and 2.5% phenylephrine hydrochloride. Fifty recordings were averaged for light-adapted ERG measured light intensity of 1.4 log cd·s/m2 in the presence of 30 cd·s/m2 background light with inter-stimulus intervals of 0.4 seconds. Scotopic and photopic b-wave amplitudes were averaged and used to generate SEM. ERG data were presented as mean±SEM. Statistical analysis was performed with unpaired t-test and significance was defined as a P value of less than 0.05.
Eyes were enucleated at six months following SR injection. Retinal sections and whole mounts were prepared according to previously described methods[28,30]. Briefly, the limbus of eyes was marked at "12 o'clock" with a hot needle immediately after sacrifice. Then eyes were enucleated and fixed in 4% paraformaldehyde overnight at 4 °C. The cornea and lens were removed from the eyes. For retinal frozen sections, the remaining eyecups were rinsed with PBS and cryoprotected 30% sucrose in PBS for 4 hours at room temperature, then embedded in cryostat compound (Tissue TEK OCT, Sakura Finetek USA, Inc., USA) and frozen at −80 °C. For immunohistochemistry, retina sections or whole mounts were rinsed in PBS and blocked in 0.3% Triton X-100, 5% BSA in PBS for 60 minutes at room temperature. Sections or whole mounts were incubated with lectin peanut agglutinin (PNA) conjugated to an Alexa Fluor 488 (1:200, Invitrogen, USA), M- or S-cone opsin (1:400, Millipore, USA), human CNGB3 (1:100, ThermoFisher, USA) primary antibodies diluted in 5% BSA in PBS overnight at 4 °C, then washed by PBS for three times, incubated with IgG secondary antibody tagged with Alexa-594 diluted 1:500 in PBS at room temperature for two hours and washed with PBS. Sections were mounted with Vectashield Mounting Medium for Fluorescence (H-1400, Vector Laboratories, USA) and coverslipped. Whole mounts and sections were analyzed with a Zeiss CD25 microscope fitted with Axiovision Rel. 4.6 software.
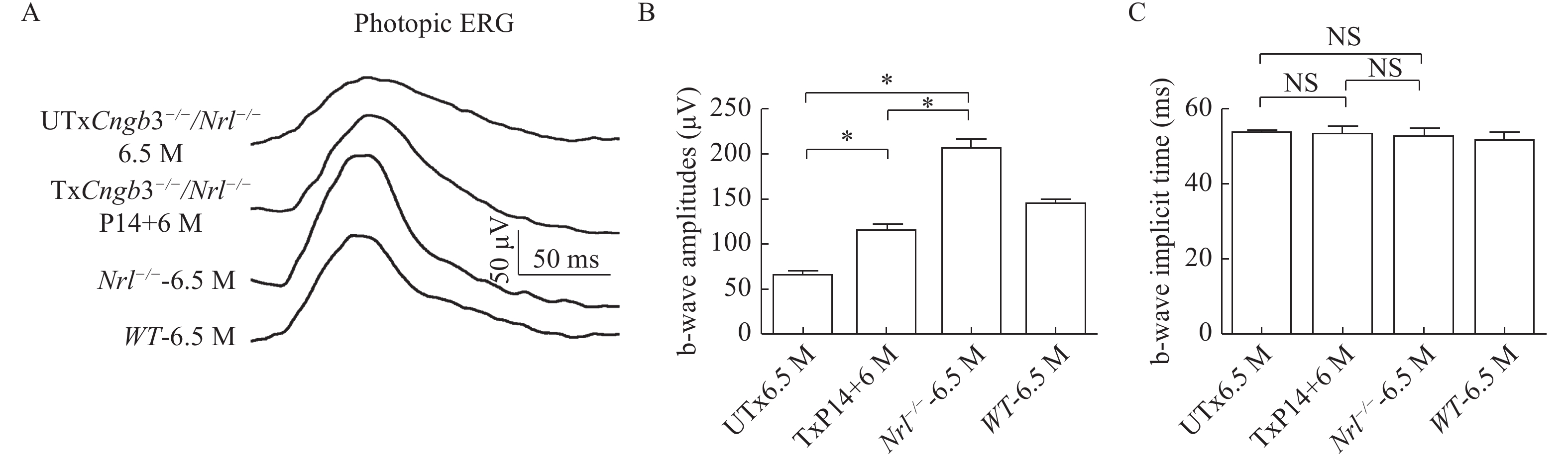
It has been shown that knock-out of Nrl gene leads to no rod-mediated ERG[16]. Therefore, both Cngb3−/−/Nrl−/− and Nrl−/− mice had no rod-mediated ERG response. The Nrl gene knock-out also caused a higher cone-mediated ERG response[31]. Thus we first tested the photopic ERGs at 6.5 months in untreated Cngb3−/−/Nrl−/− and Nrl−/− mice (ERG traces are shown in Fig. 1A). At the light intensity of 1.4 log cd·s/m2, the average b-wave amplitude record from Cngb3−/−/Nrl−/− eyes (97.86±4.92, n=5, Fig. 1B) was lower than that of Nrl−/− mouse (257.50±7.65, n=5, Fig. 1B), certified that the B subunits are not the primary subunits for the function of photoreceptor CNG channels. We also measured b-wave kinetics which was determined by b-wave implicit time of the photopic ERGs. The average b-wave implicit time which recorded from the Cngb3−/−/Nrl−/− (50.72±1.67, n=5, Fig. 1C) and Nrl−/− mice (51.94±1.68, n=5, Fig. 1C) had no statistical differences.
Since the reductions of photopic ERG in the Cngb3−/−/Nrl−/− mice, we also tested the AAV treated Cngb3−/−/Nrl−/− mice at 6 months following P14 SR injections. The b-wave amplitudes of photopic ERGs which recorded from AAV treated eyes (148.00±9.47 n=5, Fig. 1B) were significantly higher than that of untreated eyes (97.86±4.92, n=5, Fig. 1B) but were somewhat reduced compared to age-matched Nrl−/− mice (257.50±7.65, n=5, Fig. 1B). The b-wave kinetics still had no statistical difference between the treated (52.52±1.81, n=5, Fig. 1C) and the untreated (50.72±1.67, n=5, Fig. 1C) eyes.
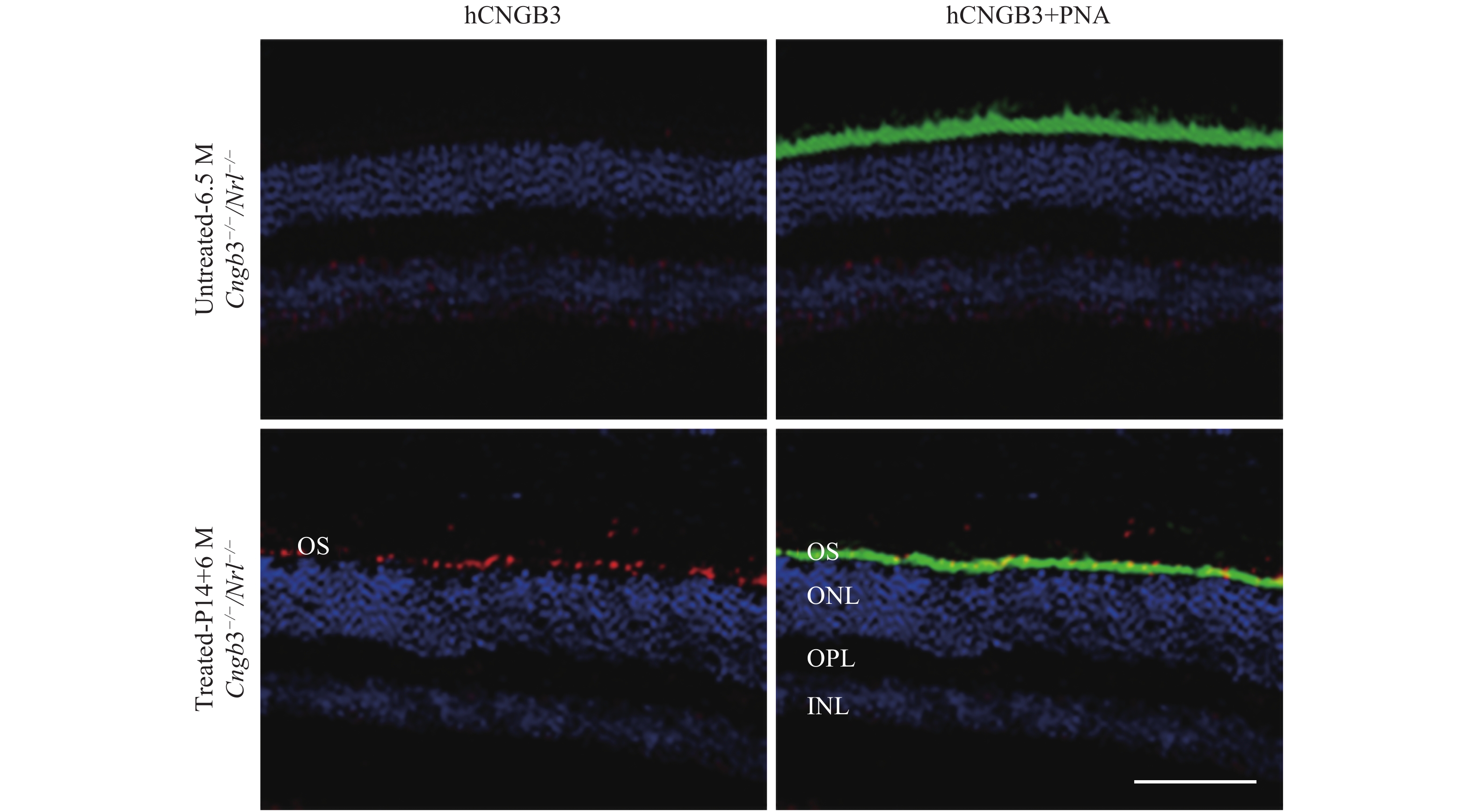
Human CNGB3 expression was assayed by immunohistochemistry at six months after SR vector treatment (Fig. 2). Robust outer segment (OS) CNGB3 expression was detected in Cngb3−/−/Nrl−/− eyes following SR-AAV8 (Y447, 733F) treatment, whereas the contralateral untreated eyes lacked detectible CNGB3 labeling (Fig. 2, left two rows). Co-localization of human CNGB3 and cone-specific PNA (Fig. 2, right two rows) confirmed that human CNGB3 expression was localized to cone outer segments.
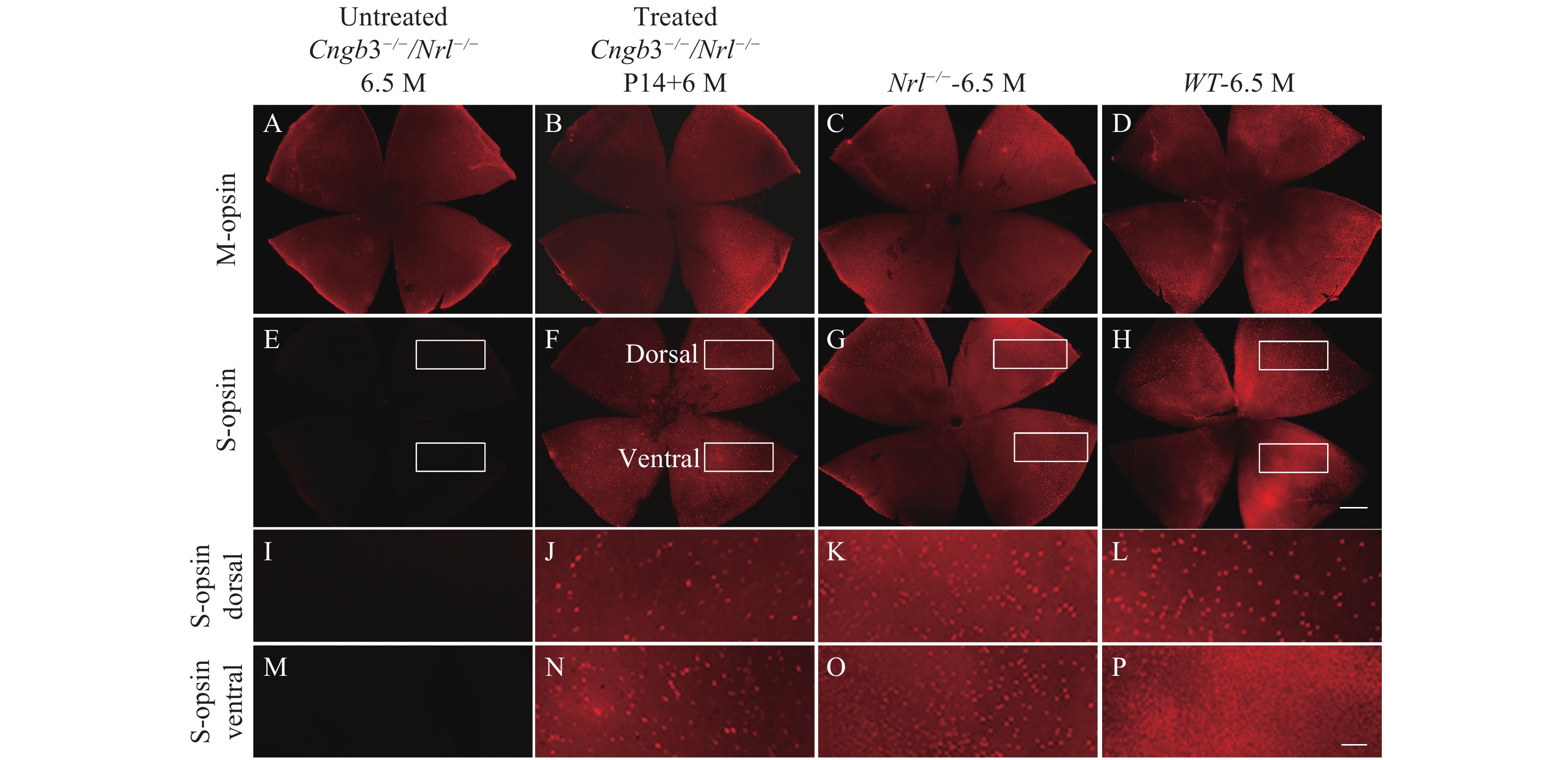
We characterized the M- and S-opsin expression in the untreated Cngb3−/−/Nrl−/− eyes by immunohistochemistry. Immunohistochemistry of the retinal whole mounts detected no S-opsin expression (Fig. 3E) while no significant reduction of M-opsin expression was observed in the untreated Cngb3−/−/Nrl−/− eyes (Fig. 3A–D). At 6.5 months of age, S-opsin expressed with the characteristic dorsal-ventral gradient in C57BL/6J retina (Fig. 3H, L and P) but throughout the Nrl−/− retinal whole mounts (Fig. 3G, K and O)[32]. M-opsin expression from untreated Cngb3−/−/Nrl−/− whole mounts also showed very similar pattern as that in the Nrl−/− retinas (Fig. 3A and C). M-cone distribution and expression intensity were similar in 6.5 months old Cngb3−/−/Nrl−/− and age-matched Nrl−/− whole mounts indicating that Cngb3−/−/Nrl−/− exhibited no significant M-cone degeneration at this age (Fig. 3A and C), whereas S-opsins were absent in Cngb3−/−/Nrl−/− mice (Fig. 3E, I and M) indicating a progressive loss of cone photoreceptors.
Expression of the S-opsin in the treated Cngb3−/−/Nrl−/− eyes was detected across the entire retinas by whole mount immunostaining and maintained at least 6 months after P14 SR injection of AAV8 (Y447, 733F) vector with therapeutic gene (Fig. 3F, J and N). Immunostaining of ventral retinal sections was also performed to check the cellular localization of S-opsin expression. In addition, S-opsin expression in the cone outer segments was evidenced by co-localization with positive PNA staining (Fig. 4). The positive S-opsin staining confirmed that SR-AAV8 (Y447, 733) treatment prevented cone degeneration (Fig. 3).
The goal of this study was to test gene therapy using a new all-cone mouse model for treating human CNGB3-achromatopsia. In the human retina, cones account for only ~5% of the photoreceptors[33]. They are primarily concentrated in macula and responsible for day vision, central visual acuity and color vision[7]. Both cone and rod CNG channels exhibit a 3:1 stoichiometry between A and B subunits[3– 5,13]. The cone photoreceptor CNG channel is composed of A3 and B3 subunits, whereas the rod photoreceptor CNG channel is formed by A1 and B1 subunits[4–6,15]. CNGB3 is known as the modulator. A CNGA3 homomeric channel is fully functional in heterologous expression systems[14,34]. Although CNGB3 shares a common topology with CNGA3 and possesses a pore-forming region, the B3 subunit does not form a functional channel without A3 subunits[14,34]. Therefore, Cnga3−/− mice show a complete loss of cone response[23], while deficiency of Cngb3 leads to residual cone response in mouse retina[35]. Fortunately, mutations in CNGB3 are clinically responsible for approximately half of all cases of ACHM patients which present moderate cone degeneration[36], to permit a relatively long period for therapy intervention. In this study, AAV delivered human CNGB3 expression in this all-cone mouse model is promising for the treatment of CNGB3-achromatopsia and other cone-specific diseases.
Nrl−/− retinas played shorter cone OS than wild-type[24,31]. Knock-out of the Cngb3 and Nrl gene partially mimicked the fovea of ACHM patients. A subsequent study showed that Cngb3−/−/Nrl−/− mice had a retinal phenotype similar to the sum of their respective single knock-out counterparts, i.e. impaired cone function and cone degeneration[16]. Effective gene therapy for ACHM requires efficient transgene expression in cones. Here, the human CNGB3 introduced by AAVs and expressed in the cone OS and S-opsin were restored following AAV-mediated CNGB3 expression, and AAV-mediated expression of the human CNGB3 could restore light responses in the Cngb3−/−/Nrl−/− mice.
The AAV serotype is one of factors that contributes to an efficient transgene delivery. Although the AAV serotype 5 transduced a significantly greater number of photoreceptor cells compared with serotype 2[37], the AAV8 had higher inherent photoreceptor transduction efficiency than AAV2 or 5[37–40], making it the most suitable vector for ACHM gene therapy. Moreover, comparing with the traditional AAV vector, site-directed tyrosine to phenylanine (Y-F) mutagenesis of selected tyrosine residues on AAV capsid also increased transduction efficiency by protecting vector particles from proteasomal degradation[30,40–41].
The specific promoter is another important factor for efficient gene therapy. The PR2.1 promoter contains 2.1 kb of nucleotides, including the locus control region (LCR) and other sequences upstream of the L-opsin coding region. This promoter was reported to direct high level of human Cngb3 expression in both L/M and S cones in mouse retinas[22]. Accordingly, we designed AAV8 (Y447, 733F)-PR2.1-hCNGB3 vector and obtained a robust and longer-term rescue in the Cngb3−/−/Nrl−/− mouse.
Our study showed very slow cone degeneration in the Cngb3−/−/Nrl−/− mice, and the photopic b-wave amplitudes were still 38% of Nrl−/− mice at the age of 6.5 months (Fig. 1B). The factor to consider was the detachment of the fragile structure of retinas after SR injection. In LCA2 human clinical trial, SR injections that detached the fovea area resulted in loss of fovea cone OS and cone cell death, and it was concluded that SR injection under the fovea may cause damage rather than benefit and should be approached with prudence[18,42–43]. Since IV delivered AAV8 (Y447, 733F) could efficiently target cone photoreceptors and rescued the cone functions in Cngb3−/−/Nrl−/−[44], we expect similar cone rescue in Cngb3−/−/Nrl−/− mice by AAV8 (Y447, 733F)-PR2.1-hCNGB3 vector. We even expect it to be utilized in clinical trials due to the reduced physical barrier to vectors intended for cones from the vitreous.
In summary, AAV-mediated human CNGB3 expression restored cone function in Cngb3−/−/Nrl−/− mouse which can be served as a good model for further study of gene therapy on CNGB3-achromatopsia.
This work was supported by NIH (Grant No. EY023543 to J.P.), Jiangsu Province Foundation for Innovative Research Team (to C.Z.).
| [1] |
Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod[J]. Annu Rev Physiol, 2006, 68: 375–401. doi: 10.1146/annurev.physiol.68.040104.134728
|
| [2] |
Pifferi S, Boccaccio A, Menini A. Cyclic nucleotide-gated ion channels in sensory transduction[J]. FEBS Lett, 2006, 580(12): 2853–2859. doi: 10.1016/j.febslet.2006.03.086
|
| [3] |
Peng CH, Rich ED, Varnum MD. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels[J]. Neuron, 2004, 42(3): 401–410. doi: 10.1016/S0896-6273(04)00225-9
|
| [4] |
Shuart NG, Haitin Y, Camp SS, et al. Molecular mechanism for 3: 1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels[J]. Nat Commun, 2011, 2: 457. doi: 10.1038/ncomms1466
|
| [5] |
Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit[J]. Neuron, 2002, 36(5): 891–896. doi: 10.1016/S0896-6273(02)01099-1
|
| [6] |
Zhong HN, Molday LL, Molday RS, et al. The heteromeric cyclic nucleotide-gated channel adopts a 3A: 1B stoichiometry[J]. Nature, 2002, 420(6912): 193–198. doi: 10.1038/nature01201
|
| [7] |
Kohl S, Baumann B, Rosenberg T, et al. Mutations in the cone photoreceptor G-protein α-subunit gene GNAT2 in patients with achromatopsia[J]. Am J Hum Genet, 2002, 71(2): 422–425. doi: 10.1086/341835
|
| [8] |
Kohl S, Varsanyi B, Antunes GA, et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia[J]. Eur J Hum Genet, 2005, 13(3): 302–308. doi: 10.1038/sj.ejhg.5201269
|
| [9] |
Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels[J]. Physiol Rev, 2002, 82(3): 769–824. doi: 10.1152/physrev.00008.2002
|
| [10] |
Kohl S, Marx T, Giddings I, et al. Total colourblindness is caused by mutations in the gene encoding theα-subunit of the cone photoreceptor cGMP-gated cation channel[J]. Nat Genet, 1998, 19(3): 257–259. doi: 10.1038/935
|
| [11] |
Thiadens AAHJ, Slingerland NWR, Roosing Ing S, et al. Genetic etiology and clinical consequences of complete and incomplete achromatopsia[J]. Ophthalmology, 2009, 116(10): 1984–1989.e1. doi: 10.1016/j.ophtha.2009.03.053
|
| [12] |
Zobor D, Zobor G, Kohl S. Achromatopsia: on the doorstep of a possible therapy[J]. Ophthalm Res, 2015, 54(2): 103–108. doi: 10.1159/000435957
|
| [13] |
Chen TY, Peng YW, Dhallan RS, et al. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods[J]. Nature, 1993, 362(6422): 764–767. doi: 10.1038/362764a0
|
| [14] |
Gerstner A, Zong XG, Hofmann F, et al. Molecular cloning and functional characterization of a new modulatory cyclic nucleotide-gated channel subunit from mouse retina[J]. J Neurosci, 2000, 20(4): 1324–1332. doi: 10.1523/JNEUROSCI.20-04-01324.2000
|
| [15] |
Schön C, Biel M, Michalakis S. Gene replacement therapy for retinal CNG channelopathies[J]. Mol Genet Genom, 2013, 288(10): 459–467. doi: 10.1007/s00438-013-0766-4
|
| [16] |
Thapa A, Morris L, Xu JH, et al. Endoplasmic reticulum stress-associated cone photoreceptor degeneration in cyclic nucleotide-gated channel deficiency[J]. J Biol Chem, 2012, 287(22): 18018–18029. doi: 10.1074/jbc.M112.342220
|
| [17] |
Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial[J]. Lancet, 2009, 374(9701): 1597–1605. doi: 10.1016/S0140-6736(09)61836-5
|
| [18] |
Jacobson SG, Cideciyan AV, Ratnakaram R, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years[J]. Arch Ophthalmol, 2012, 130(1): 9–24. doi: 10.1001/archophthalmol.2011.298
|
| [19] |
Bainbridge JWB, Mehat MS, Sundaram V, et al. Long-term effect of gene therapy on Leber's congenital amaurosis[J]. N Engl J Med, 2015, 372(20): 1887–1897. doi: 10.1056/NEJMoa1414221
|
| [20] |
Ye GJ, Komáromy AM, Zeiss C, et al. Safety and efficacy of AAV5 vectors expressing human or canine CNGB3 in CNGB3-mutant dogs[J]. Hum Gene Ther Clin Dev, 2017, 28(4): 197–207. doi: 10.1089/humc.2017.125
|
| [21] |
Carvalho LS, Xu JH, Pearson RA, et al. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy[J]. Hum Mol Genet, 2011, 20(16): 3161–3175. doi: 10.1093/hmg/ddr218
|
| [22] |
Ye GJ, Budzynski E, Sonnentag P, et al. Cone-specific promoters for gene therapy of achromatopsia and other retinal diseases[J]. Hum Gene Ther, 2016, 27(1): 72–82. doi: 10.1089/hum.2015.130
|
| [23] |
Biel M, Seeliger M, Pfeifer A, et al. Selective loss of cone function in mice lacking the cyclic nucleotide-gated channel CNG3[J]. Proc Natl Acad Sci USA, 1999, 96(13): 7553–7557. doi: 10.1073/pnas.96.13.7553
|
| [24] |
Mears AJ, Kondo M, Swain PK, et al. Nrl is required for rod photoreceptor development[J]. Nat Genet, 2001, 29(4): 447–452. doi: 10.1038/ng774
|
| [25] |
Alexander JJ, Umino Y, Everhart D, et al. Restoration of cone vision in a mouse model of achromatopsia[J]. Nat Med, 2007, 13(6): 685–687. doi: 10.1038/nm1596
|
| [26] |
Zolotukhin S, Potter M, Zolotukhin I, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors[J]. Methods, 2002, 28(2): 158–167. doi: 10.1016/S1046-2023(02)00220-7
|
| [27] |
Qi Y, Dai XF, Zhang H, et al. Trans-corneal subretinal injection in mice and its effect on the function and morphology of the retina[J]. PLoS One, 2015, 10(8): e0136523. doi: 10.1371/journal.pone.0136523
|
| [28] |
Pang JJ, Boye SL, Kumar A, et al. AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEβ mutation[J]. Invest Ophthalmol Vis Sci, 2008, 49(10): 4278–4283. doi: 10.1167/iovs.07-1622
|
| [29] |
Pang JJ, Chang B, Kumar A, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis[J]. Mol Ther, 2006, 13(3): 565–572. doi: 10.1016/j.ymthe.2005.09.001
|
| [30] |
Pang JJ, Dai XF, Boye SE, et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa[J]. Mol Ther, 2011, 19(2): 234–242. doi: 10.1038/mt.2010.273
|
| [31] |
Daniele LL, Lillo C, Lyubarsky AL, et al. Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse[J]. Invest Ophthalmol Vis Sci, 2005, 46(6): 2156–2167. doi: 10.1167/iovs.04-1427
|
| [32] |
Oh ECT, Cheng H, Hao H, et al. Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors[J]. Brain Res, 2008, 1236: 16–29. doi: 10.1016/j.brainres.2008.01.028
|
| [33] |
Curcio CA, Allen KA, Sloan KR, et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin[J]. J Comp Neurol, 1991, 312(4): 610–624. doi: 10.1002/cne.903120411
|
| [34] |
Faillace MP, Bernabeu RO, Korenbrot JI. Cellular processing of cone photoreceptor cyclic GMP-gated ion channels: a role for the S4 structural motif[J]. J Biol Chem, 2004, 279(21): 22643–22653. doi: 10.1074/jbc.M400035200
|
| [35] |
Ding XQ, Harry CS, Umino Y, et al. Impaired cone function and cone degeneration resulting from CNGB3 deficiency: down-regulation of CNGA3 biosynthesis as a potential mechanism[J]. Hum Mol Genet, 2009, 18(24): 4770–4780. doi: 10.1093/hmg/ddp440
|
| [36] |
Genead MA, Fishman GA, Rha J, et al. Photoreceptor structure and function in patients with congenital achromatopsia[J]. Invest Ophthalmol Vis Sci, 2011, 52(10): 7298–7308. doi: 10.1167/iovs.11-7762
|
| [37] |
Yang GS, Schmidt M, Yan ZY, et al. Virus-mediated transduction of murine retina with adeno-associated virus: effects of viral capsid and genome size[J]. J Virol, 2002, 76(15): 7651–7660. doi: 10.1128/JVI.76.15.7651-7660.2002
|
| [38] |
Stieger K, Colle MA, Dubreil L, et al. Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain[J]. Mol Ther, 2008, 16(5): 916–923. doi: 10.1038/mt.2008.41
|
| [39] |
Natkunarajah M, Trittibach P, McIntosh J, et al. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8[J]. Gene Ther, 2008, 15(6): 463–467. doi: 10.1038/sj.gt.3303074
|
| [40] |
Petrs-Silva H, Dinculescu A, Li QH, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors[J]. Mol Ther, 2009, 17(3): 463–471. doi: 10.1038/mt.2008.269
|
| [41] |
Zhong L, Li BZ, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses[J]. Proc Natl Acad Sci USA, 2008, 105(22): 7827–7832. doi: 10.1073/pnas.0802866105
|
| [42] |
Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial[J]. Hum Gene Ther, 2008, 19(10): 979–990. doi: 10.1089/hum.2008.107
|
| [43] |
Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis[J]. N Engl J Med, 2008, 358(21): 2240–2248. doi: 10.1056/NEJMoa0802315
|
| [44] |
Du W, Tao Y, Deng WT, et al. Vitreal delivery of AAV vectored Cnga3 restores cone function in CNGA3-/-/Nrl-/- mice, an all-cone model of CNGA3 achromatopsia[J]. Hum Mol Genet, 2015, 24(13): 3699–3707.
|
| [1] | Xiaoxiao Cao, Wenhao Zhu, Zhenghan Luo, Ran He, Yihao Li, Shirong Hui, Sheng Yang, Rongbin Yu, Peng Huang. The association between weekly mean temperature and the epidemic of influenza across 122 countries/regions, 2014–2019[J]. The Journal of Biomedical Research. DOI: 10.7555/JBR.39.20250010 |
| [2] | Tiwari-Heckler Shilpa, Jiang Z. Gordon, Popov Yury, J. Mukamal Kenneth. Daily high-dose aspirin does not lower APRI in the Aspirin-Myocardial Infarction Study[J]. The Journal of Biomedical Research, 2020, 34(2): 139-142. DOI: 10.7555/JBR.33.20190041 |
| [3] | Huan Liu, Shijiang Zhang, Yongfeng Shao, Xiaohu Lu, Weidong Gu, Buqing Ni, Qun Gu, Junjie Du. Biomechanical characterization of a novel ring connector for sutureless aortic anastomosis[J]. The Journal of Biomedical Research, 2018, 32(6): 454-460. DOI: 10.7555/JBR.31.20170011 |
| [4] | Minbo Zang, Qiao Zhou, Yunfei Zhu, Mingxi Liu, Zuomin Zhou. Effects of chemotherapeutic agent bendamustine for nonhodgkin lymphoma on spermatogenesis in mice[J]. The Journal of Biomedical Research, 2018, 32(6): 442-453. DOI: 10.7555/JBR.31.20170023 |
| [5] | Kaibo Lin, Shikun Zhang, Jieli Chen, Ding Yang, Mengyi Zhu, Eugene Yujun Xu. Generation and functional characterization of a conditional Pumilio2 null allele[J]. The Journal of Biomedical Research, 2018, 32(6): 434-441. DOI: 10.7555/JBR.32.20170117 |
| [6] | Huanqiang Wang, Congying Yang, Siyuan Wang, Tian Wang, Jingling Han, Kai Wei, Fucun Liu, Jida Xu, Xianzhen Peng, Jianming Wang. Cell-free plasma hypermethylated CASZ1, CDH13 and ING2 are promising biomarkers of esophageal cancer[J]. The Journal of Biomedical Research, 2018, 32(6): 424-433. DOI: 10.7555/JBR.32.20170065 |
| [7] | Fengzhen Wang, Mingwan Zhang, Dongsheng Zhang, Yuan Huang, Li Chen, Sunmin Jiang, Kun Shi, Rui Li. Preparation, optimization, and characterization of chitosancoated solid lipid nanoparticles for ocular drug delivery[J]. The Journal of Biomedical Research, 2018, 32(6): 411-423. DOI: 10.7555/JBR.32.20160170 |
| [8] | Trudy M. Forte, Vineeta Sharma, Robert O. Ryan. Apolipoprotein A-V gene therapy for disease prevention / treatment:a critical analysis[J]. The Journal of Biomedical Research, 2016, 30(2): 88-93. DOI: 10.7555/JBR.30.20150059 |
| [9] | Xinting Pan, Liqun Wu, Jingyu Cao, Weidong Guo, Zusen Wang, Bing Han, Weiyu Hu. Recombinant adenovirus vector-mediated human MDA-7 gene transfection suppresses hepatocellular carcinoma growth in a mouse xenograft model[J]. The Journal of Biomedical Research, 2012, 26(1): 53-58. DOI: 10.1016/S1674-8301(12)60007-4 |
| [10] | Zhengxian Tao, Bo Chen, Yingming Zhao, Hongwu Chen, Liansheng Wang, Yonghong Yong, Kejiang Cao, Qifeng Yu, Danian Ke, Hua Wang, Zuze Wu, Zhijian Yang. HGF percutaneous endocardial injection induces cardiomyocyte proliferation and rescues cardiac function in pigs[J]. The Journal of Biomedical Research, 2010, 24(3): 198-206. |
| 1. | Baxter MF, Borchert GA. Gene Therapy for Achromatopsia. Int J Mol Sci, 2024, 25(17): 9739. DOI:10.3390/ijms25179739 |
| 2. | Crane R, Conley SM, Al-Ubaidi MR, et al. Gene Therapy to the Retina and the Cochlea. Front Neurosci, 2021, 15: 652215. DOI:10.3389/fnins.2021.652215 |