| Citation: | Xiaolong Zheng, Wei Wang. Astrocyte transplantation for repairing the injured spinal cord[J]. The Journal of Biomedical Research, 2022, 36(5): 312-320. DOI: 10.7555/JBR.36.20220012 |
Traumatic spinal cord injury (SCI) leads to permanent neurological deficits in motor, sensory and autonomic function that cannot be cured by current treatments[1], which places a substantial burden on individuals, families and society[2]. Histologically, the spinal cord is composed of many cells and structures, including neurons, glia, axons and myelin, all of which are lost in the injury epicenter after the spinal cord damage, producing a large cavity[3]. Thus, transplanting exogenous cells to replace the lost cells is a promising strategy to repair the injured spinal cord. In the past 30 years, many kinds of cells have been experimentally transplanted to treat SCI[4–5], including Schwann cells, olfactory ensheathing cells (OECs), mesenchymal stromal cells (MSCs), neural progenitor cells (NPCs), and oligodendrocyte progenitor cells (OPCs). These grafted cells partially promote functional recovery by several mechanisms, such as neuroprotection, immunomodulation, axon regeneration and sprouting, relay formation and remyelination[4–5]. Of note, NPCs and OPCs are receiving superior attention to other cells for transplantation in that NPCs produce neurons that can form relay circuits[6], and OPCs produce oligodendrocytes that can remyelinate axons[7–9], both of which are unable to regenerate spontaneously after SCI. However, there are still challenges limiting the effect of grafting NPCs and OPCs for treating SCI, including poor graft survival, lack of functional synaptic connections, and myelin formation.
Astrocytes are abundant glia in the spinal cord that structurally and functionally support the normal function of neurons[10]. After the spinal cord is injured, astrocytes proliferate and become active, forming extensive scars and secreting extracellular matrix surrounding the lesion. Reactive astrocytes have long been regarded as a barrier for axonal regeneration after SCI[11]; however, recent progress has uncovered the beneficial role of astrocytes in repairing lesioned spinal cords. For example, the abolishment of reactive astrocytes after spinal crush injury in mice causes widespread tissue disruption, pronounced cellular degeneration, and failure of wound contraction, with severe persisting motor deficits[12–13]. Moreover, spontaneous regrowth of transected corticospinal, sensory, or serotonergic axons through severe SCI lesions failed after preventing reactive astrocyte formation[14]. Thus, astrocytes could be manipulated to reshape the microenvironment of the lesion to harness benefits in the repair of injured spinal cord. In addition, astrocytes could be added to neuron grafts[15] since astrocytes could support neuron maturation[16] and enhance synaptic plasticity[17]. Astrocytes also enhance oligodendrogenesis or remyelination[18]. In the past three decades, studies on astrocyte transplantation for SCI treatment have been carried out and the progress has been summarized in several excellent reviews[19–24]. However, the proper origins of astrocytes for SCI treatment remain unclear. Here, we reintroduce this field from a different perspective with the aim to maximize the beneficial effect of astrocyte transplantation in spinal cord repair.
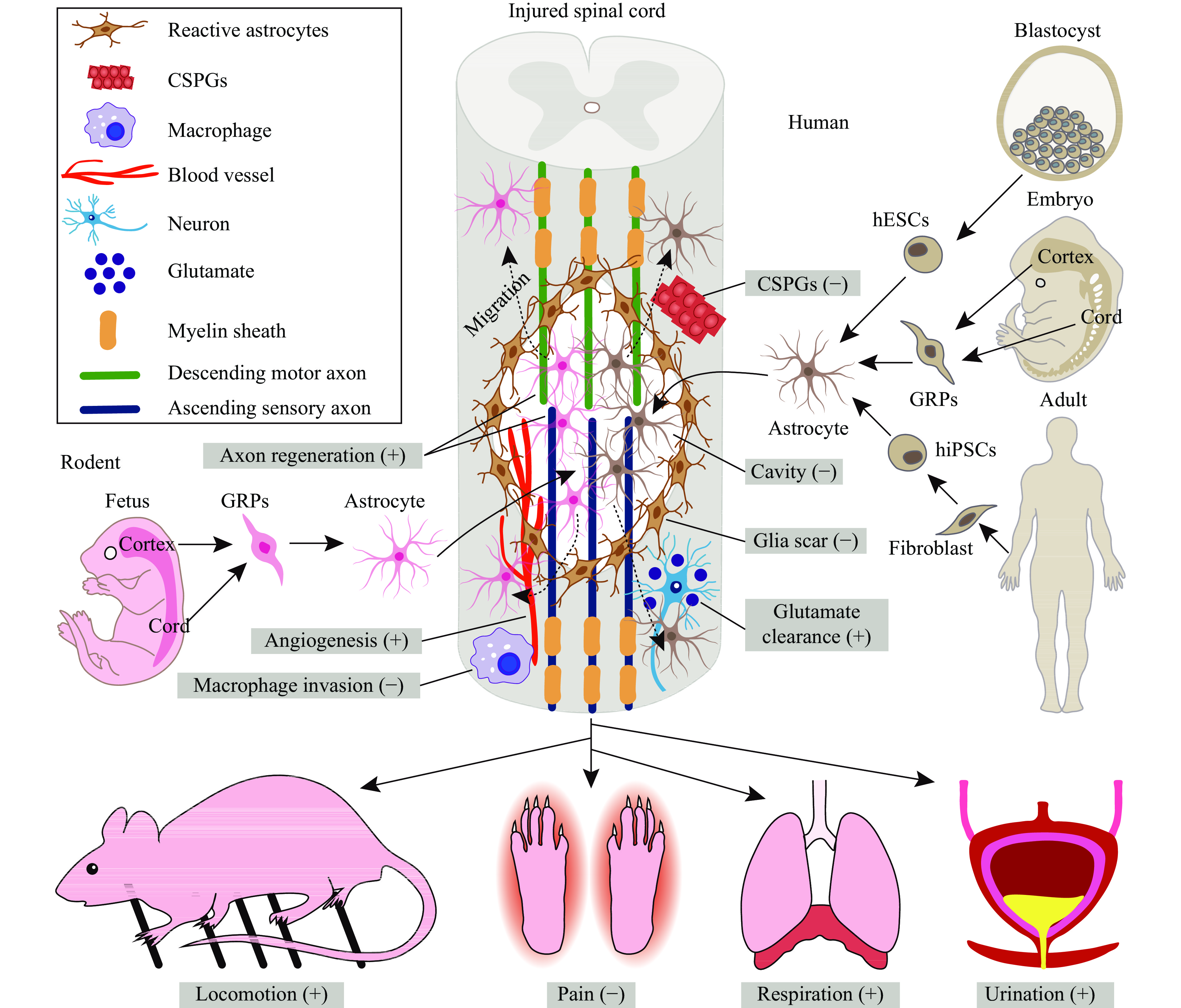
Because of their easy availability and rapid maturation, rodent astrocytes were initially transplanted to test their efficacy in repairing spinal lesions and promoting functional recovery (Fig. 1). These grafted rodent astrocytes are primarily derived from both the embryonic and postnatal brain and spinal cord (Fig. 1), which means that they are in fact immature progenitors in development. When grafted into the lesioned spinal cord, these cells survive, migrate and mature, promoting motor, sensory, respiratory and autonomic functional recovery (Fig. 1).
The key to cell transplantation in SCI is the survival and differentiation of grafted cells. When glial restricted progenitors (GRPs) derived from the embryonic rat spinal cord are grafted into the naïve spinal cord, these cells survive well for at least 6 weeks, differentiate into nearly pure astrocytes, and migrate extensively in the white matter both rostrally and caudally[25]. Based on this evidence, these GRPs are again grafted into lateral funiculus transection lesions and survive the lesion environment for at least 5 weeks, having the same differentiation and migration pattern as when grafted into the naïve spinal cord[25]. Furthermore, when neuronal restricted progenitors (NRPs) are added to GRP grafts, they even completely fill the lesion[26]; when these GRPs are pretreated with different factors, all of them show equivalent survival and differentiation[27]. However, when the same GRPs are grafted into the contusion injury, they only survive within the parenchyma around the cavity, and no cells present within the cavity[28]. In addition to spinal astrocytes, cortical astrocytes derived from both postnatal[29] and adult[30] rats can also survive and migrate when grafted into spinal hemisection injury. In summary, astrocytes with different origins can survive a variety of spinal injuries for a relatively long period, paving the way for their potential in SCI treatment.
The notion for grafting astrocytes to cure SCI is to promote axon regrowth. Initially, embryonic rat spinal cord-derived GRPs have been found to stimulate the regrowth of crushed dorsal root axons into the dorsal column and gray matter[31], and they can also induce regrowth of the transected ascending dorsal column sensory axons into grafts[27]; however, these axons never grow out beyond the transected lesion. For the descending motor axons, the corticospinal tract (CST) and the raphespinal tract seem to contact these GRPs grafted into contusion lesions; however, these tracts still cannot regenerate out of the lesion cavity[28]. In fact, CST fails to regrow into the grafted GRPs[32]. In addition to spinal GRPs, neocortical astrocytes can also stimulate the growth of lesioned axons into grafts[29,33–34]; yet, the kinds of axons that grow remain unclear. Besides regeneration, the grafted astrocytes exert other effects on the injured host spinal cord; they can inhibit host reactive scar formation[28–29] and reduce the lesion cavity[29]. In addition, macrophage infiltration is alleviated[28], secretion of axon growth inhibiting extracellular matrix is reduced[28], and angiogenesis is enhanced[33]. All these reactions can partly explain the beneficial effects of transplanting astrocytes for treating SCI.
Regaining motor control is essential for the SCI population to achieve primary functional recovery[35–36]. Initially, grafting embryonic spinal astrocytes into dorsal column transection significantly worsens hindlimb fine motor control[37]. In addition, grafted embryonic spinal GRPs[38] or GRP-derived astrocytes with ciliary neurotrophic factor (CNTF) differentiation (GDAsCNTF)[39] deposit many chondroitin sulfate proteoglycans (CSPGs), preventing the growth of both dorsal column axons and the rubrospinal tract, and failing to improve fine motor control[38–39]. However, when grafting GRP-derived astrocytes with bone morphogenetic protein (BMP) differentiation (GDAsBMP), surprisingly, the dorsal column axons and rubrospinal tract can robustly regenerate into grafts and beyond the lesion, and fine motor control is significantly improved[38], probably due to less secreted CSPGs and realignment of host astrocyte processes in parallel enabling axon regeneration[38]. Unfortunately, neither GRPs nor GDAsBMP could preserve host spinal tissue or improve hindlimb locomotion when grafted subacutely after contusion lesion[40], but once immediately grafted after injury, GDAsBMP could preserve more corticospinal and sensory axons and improve hindlimb locomotion[41]. Additionally, GDAsBMP engineered to express multineurotrophin D15A[40] or supplemented with human recombinant decorin[41] can significantly increase spinal tissue preservation and hindlimb locomotion. Moreover, when GRPs are mixed into NRPs and cografted, axons, including 5-hydroxytryptamine (5-HT) fibers, can regenerate with significantly elevated hindlimb general locomotion[42]; however, improved fine motor control of hindlimbs cannot be observed[42]. In addition to GRPs, neocortical astrocytes can promote the growth of many axons after dorsal hemisection[43] or compression[44], and improve general locomotion but fail to enhance fine motor control.
Sensory function, particularly neuropathic pain after SCI, is often neglected in cell transplantation research. This is very important since grafted cells can aggravate pain[45–46]. In fact, grafting of GRPs or GDAsCNTF leads to both mechanical and thermal allodynia as early as 3 weeks post dorsal column transection[39]; in contrast, allodynia does not develop in GDAsBMP grafts[39], while significantly increased aberrant sprouting of calcitonin gene-related peptide (CGRP) in the dorsal horn is only present in GRPs or GDAsCNTF grafts but not in GDAsBMP grafts[39]. Interestingly, when GRPs or GDAsBMP are grafted into contusion injury, mechanical allodynia does not develop[40]. Moreover, when GRPs are mixed into NRPs and cografted, thermal hypersensitivity is diminished as early as one-week post grafting, and the sprouting of CGRP fibers is inhibited in the dorsal horn[42]. Grafting of mouse-induced pluripotent stem cells (iPSCs) differentiated astrocytes into contusion lesions can induce mechanical but not thermal allodynia; however, aberrant CGRP fiber sprouting does not increase in the dorsal horn[47]. In addition, grafting neocortical astrocytes fails to cause any apparent signs of increased pain sensitivity or the presence of chronic pain or self-mutilation[44].
SCI often occurs in the cervical spine, causing breath distress, and some studies have tried to find treatments, including astrocyte transplantation. Grafting GRPs into cervical hemisection injury not only stimulates robust regeneration of the ipsilateral bulbospinal respiratory tract and 5-HT fibers but also induces sprouting of the contralateral bulbospinal respiratory tract. Electromyogram recordings from the ipsilateral hemidiaphragm revealed significantly increased burst amplitudes, demonstrating substantial recovery of diaphragm function[48]. However, when GDAsBMP are grafted into cervical hemicontusion injury, no improvement in diaphragm function is observed[49]; if GDAsBMP are engineered to overexpress glutamate transporter 1 (GLT-1) to enhance glutamate uptake, the lesion volume, number of phrenic motor neurons and diaphragm innervation are rescued, and diaphragm function is improved[49].
SCI also leads to autonomic dysfunction, including bladder dysfunction, which severely affects the quality of life; however, few studies have attempted to address this problem with astrocyte transplantation. When GRPs are mixed with NRPs and cografted into contusion injury, accelerated recovery of bladder contraction from the spinal shock phase and increased voiding efficiency are observed as early as 2 weeks post grafting. At 8 weeks after transplantation, urodynamic parameters, including micturition pressure, residual urine, bladder capacity, and bladder weight, are reduced[42].
Though rodent astrocyte transplantation shows promising beneficial results in animal models of SCI, it cannot be used to treat human SCI because of xenotransplantation. Instead, human astrocytes should be grafted; however, preclinical research should be done before they enter clinical practice. To date, human astrocytes from several origins have shown promise in treating SCI (Fig. 1).
While the most commonly used rodent astrocytes in spinal repair are of fetal spinal cord origin, the more studied human fetal astrocytes for treating SCI were obtained from the brain. Direct transplantation of human fetal brain GRPs in spinal hemisection sites can induce regeneration of both the ascending dorsal column sensory axons and descending motor axons, including reticulospinal and raphespinal tracts, while other descending motor axons, such as coerulospinal and rubrospinal tracts, did not show any increased regrowth[50]. Further differentiation of human fetal brain GRPs into astrocytes of different maturities with BMP or CNTF also results in equal survival in dorsal column transection lesions and promoted similar growth of ascending sensory axons[51]. However, this is not the case in spinal contusion injury. Although direct grafting of human fetal brain GRPs into the contusion epicenter can reduce the lesion cavity, suppress both glial and fibrotic scar formation and aid in the regrowth of 5-HT fibers, general locomotion, fine motor control, and sensory and bladder function cannot show significant improvement[52]. In contrast, when these human fetal brain GRPs are predifferentiated into astrocytes by BMP, sensory and bladder function recover significantly, although general locomotion and fine motor control are still not improved[52].
The role of human fetal astrocytes (from the fetal spinal cord) in SCI treatment has only been reported in one study[53]. Directly grafting human fetal spinal GRPs or their derived astrocytes with CNTF differentiation after hemisection lesion can neither protect host spinal neurons from death nor improve fine motor control[53]; in contrast, only BMP-induced astrocytes can stimulate robust axon regeneration and promote host spinal neurons from death, thus significantly improving fine motor control as early as one-week post grafting[53].
The derivation of human embryonic stem cells (hESCs)[54–56] enables the differentiation of all kinds of cells in adult humans. Due to the establishment of a protocol for differentiating hESCs into neural progenitors[57–58], astrocytes can also be obtained from hESCs. Initially, grafted hESC-derived cerebral neural progenitors profoundly differentiate into mature astrocytes in the naïve spinal cord, migrating extensively and integrating structurally within the host spinal cord over 9 months[59]. When grafted into spinal hemisection lesions, nearly half of hESC-derived cerebral neural progenitors become astrocytes after 18 months, which extensively migrate both rostrally and caudally[60]. Moreover, these differentiated astrocytes suppress host reactive glial scar formation in the injury site, adopting blood–spinal cord barrier phenotypes, supporting neuronal function by regulating neurotransmitter levels, and protecting neurons from excitotoxicity[61].
In addition, spinal astrocyte progenitors can be differentiated from hESCs[62], and they survive well for at least 12 weeks in the naïve spinal cord, differentiating exclusively into astrocytes[63]. Gene profiling has revealed that these grafted human astrocytes become mature, expressing both structural and functional proteins of astrocytes, while genes related to neural progenitors, oligodendroglia, microglia, and neurons are almost absent[63]. However, it is unclear whether hESC-derived spinal astrocytes can aid in functional recovery after SCI, which merits further investigation.
The discovery of iPSCs enables the obtainment and autologous transplantation of any cells without immune rejection[64]. Specifically, the generation of human iPSCs[65–66] holds great promise in clinical translation. Human spinal astrocytes with high purity can be obtained from human iPSCs[62], and they can survive and mature in 12 weeks after grafting into the naïve spinal cord[63]. However, although they can survive and mature into astrocytes in spinal hemicontusion lesions, they fail to improve respiratory function[67]. This was probably due to the absence of GLT-1 expression in human iPSC-derived astrocytes, since overexpression of GLT-1 enables high glutamate uptake, significantly reducing the lesion area, preserving the innervation of the diaphragm, and improving respiratory function[67].
Although transplantation of human iPSC-derived astrocytes is promising in treating neurological disorders, the potential safety concerns prevent their use in clinical practice. For example, grafting human iPSC-derived astrocytes from amyotrophic lateral sclerosis (ALS) patients into the naïve spinal cord may induce ALS-like symptoms in host mice[59], and human astrocytes with ALS may induce motor neuron degeneration in host mice[68]. Thus, the origin of human iPSCs is vital to cell transplantation.
Although recent progress in astrocyte transplantation for SCI treatment is a prospective method (Fig. 1), there are still many challenges before it enters clinical trials.
The first challenge is to ensure the sufficient amount of human astrocytes for transplantation, as the scale of humans is much more escalated than rodents; that is, one rat may need only a million cells, while more than hundreds of millions of cells are needed for an individual. Isolating enough astrocytes from the human fetal spinal cord or brain can hardly meet the demand, while directed differentiation of astrocytes from human ESCs or iPCSs can be a better solution because of their high pluripotency and proliferation. However, it takes almost 6 months to obtain functional astrocytes from hESCs[69–70], and the differentiation time is much longer than the optimal time window for cell transplantation (usually 2 to 4 weeks after injury). Therefore, rapid generation of human functional astrocytes from human ESCs and iPSCs is needed. By inducible expression of nuclear factor IA, astrocytes can be efficiently generated in 4 to 7 weeks[71–72], which tremendously accelerates the generation of functional human astrocytes in large numbers.
The second unresolved problem is whether human spinal or cerebral astrocytes, which are better for treating SCI, display diverse morphologies in different regions of the central nervous system. RNA sequencing of human pluripotent stem cell-derived regional astrocytes reveals distinct transcript profiles, suggesting differential functional properties, such as effects on neurite growth and blood-brain barrier formation[73]. There is evidence supporting the use of spinal NPCs rather than cerebral NPCs since only spinal NPCs can induce CST regeneration[32,74]. It seems that human spinal astrocytes are superior for integrating into the spinal cord and improving functional recovery; however, this needs further exploration.
In addition, in almost all current studies, astrocytes are transplanted in the acute or subacute phase after SCI, and whether human astrocytes could be used to treat chronic spinal injuries remains unclear. This is a vital problem because most individuals with spinal injuries are in the chronic phase, and patients are specifically concerned about the effects of cell transplantation on chronic SCI[75]. And the long-term effects of astrocyte transplantation on other complications related to spinal injury, such as spasms or repeated hypotensive episodes, need close observation and in-depth study because these syndromes may largely impair quality of life.
Finally, the efficacy and safety of transplanting astrocytes for treating SCI have only been tested in rodent animal models. Even though the rodent spinal cord shares many similarities with that of humans, there are still differences between them. For example, the length and diameter of the human spinal cord are much larger than those of the rat spinal cord. In addition, the CST is in the dorsal column of the rat spinal cord, while it is in the lateral column of the human spinal cord. The locomotion of rats is solely quadrupedal, while humans engage in bipedal walking. The lifetime of rodents is much shorter than that of humans, and therefore the observation period on rats is insufficient to recognize the potential unwanted side effects. In summary, the findings gained from rodents are insufficient to prove the safety and efficacy of astrocyte transplantation. This gap may be resolved by applying a large animal nonhuman primate SCI model that resembles human SCI both anatomically and physiologically[76–78] to observe the long-term safety and efficacy of astrocyte transplantation before initiating clinical trials.
Although many basic studies on astrocyte transplantation for spinal repair have been conducted, unfortunately, no clinical trial has been initiated currently. By contrast, transplantation of other cells, such as Schwann cells, OECs, MSCs, NPCs, and OPCs, have been tested in several clinical trials. This sharp difference could partly be attributed to the notion that astrocytes can self-proliferate extensively and form reactive astrogliosis, which has long been regarded as a barrier to axon regeneration; thus, transplanting exogenous astrocytes seems unnecessary or even harmful to spinal repair, making researchers reluctant to delve into the benefits of astrocyte transplantation for SCI and thus leading to insufficient evidence for the initiation of clinical trials. Thus, more studies regarding the ideal origins of astrocytes (e.g., at higher cervical lesion levels), chronic SCI phase, severe complete SCI lesions, prolonged observation periods of astrocyte transplantation, and large animal models of SCI should be conducted, which can pave the way for the clinical translation of astrocyte transplantation for SCI treatment.
CLC number: R651.2, Document code: A
The authors reported no conflict of interests.
| [1] |
Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury[J]. Nat Rev Dis Primers, 2017, 3: 17018. doi: 10.1038/nrdp.2017.18
|
| [2] |
GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016[J]. Lancet Neurol, 2019, 18(1): 56–87. doi: 10.1016/S1474-4422(18)30415-0
|
| [3] |
O'Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair[J]. J Clin Investigation, 2017, 127(9): 3259–3270. doi: 10.1172/JCI90608
|
| [4] |
Assinck P, Duncan GJ, Hilton BJ, et al. Cell transplantation therapy for spinal cord injury[J]. Nat Neurosci, 2017, 20(5): 637–647. doi: 10.1038/nn.4541
|
| [5] |
Vismara I, Papa S, Rossi F, et al. Current options for cell therapy in spinal cord injury[J]. Trends Mol Med, 2017, 23(9): 831–849. doi: 10.1016/j.molmed.2017.07.005
|
| [6] |
Fischer I, Dulin JN, Lane MA. Transplanting neural progenitor cells to restore connectivity after spinal cord injury[J]. Nat Rev Neurosci, 2020, 21(7): 366–383. doi: 10.1038/s41583-020-0314-2
|
| [7] |
Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury[J]. J Neurosci, 2005, 25(19): 4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005
|
| [8] |
Kawabata S, Takano M, Numasawa-Kuroiwa Y, et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury[J]. Stem Cell Reports, 2016, 6(1): 1–8. doi: 10.1016/j.stemcr.2015.11.013
|
| [9] |
Nagoshi N, Khazaei M, Ahlfors JE, et al. Human spinal oligodendrogenic neural progenitor cells promote functional recovery after spinal cord injury by axonal remyelination and tissue sparing[J]. Stem Cells Transl Med, 2018, 7(11): 806–818. doi: 10.1002/sctm.17-0269
|
| [10] |
Verkhratsky A, Nedergaard M. Physiology of astroglia[J]. Physiol Rev, 2018, 98(1): 239–389. doi: 10.1152/physrev.00042.2016
|
| [11] |
Silver J, Miller JH. Regeneration beyond the glial scar[J]. Nat Rev Neurosci, 2004, 5(2): 146–156. doi: 10.1038/nrn1326
|
| [12] |
Faulkner JR, Herrmann JE, Woo MJ, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury[J]. J Neurosci, 2004, 24(9): 2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004
|
| [13] |
Gu Y, Cheng X, Huang X, et al. Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair[J]. Brain Behav Immun, 2019, 80: 394–405. doi: 10.1016/j.bbi.2019.04.016
|
| [14] |
Anderson MA, Burda JE, Ren Y, et al. Astrocyte scar formation aids central nervous system axon regeneration[J]. Nature, 2016, 532(7598): 195–200. doi: 10.1038/nature17623
|
| [15] |
Song JJ, Oh SM, Kwon OC, et al. Cografting astrocytes improves cell therapeutic outcomes in a Parkinson's disease model[J]. J Clin Invest, 2018, 128(1): 463–482. doi: 10.1172/JCI93924
|
| [16] |
Hedegaard A, Monzón-Sandoval J, Newey SE, et al. Pro-maturational effects of human iPSC-derived cortical astrocytes upon iPSC-derived cortical neurons[J]. Stem Cell Reports, 2020, 15(1): 38–51. doi: 10.1016/j.stemcr.2020.05.003
|
| [17] |
Han X, Chen M, Wang F, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice[J]. Cell Stem Cell, 2013, 12(3): 342–353. doi: 10.1016/j.stem.2012.12.015
|
| [18] |
Jiang P, Chen C, Liu X, et al. Human iPSC-derived immature astroglia promote oligodendrogenesis by increasing TIMP-1 secretion[J]. Cell Reports, 2016, 15(6): 1303–1315. doi: 10.1016/j.celrep.2016.04.011
|
| [19] |
Noble M, Davies JE, Mayer-Pröschel M, et al. Precursor cell biology and the development of astrocyte transplantation therapies: lessons from spinal cord injury[J]. Neurotherapeutics, 2011, 8(4): 677–693. doi: 10.1007/s13311-011-0071-z
|
| [20] |
Chu T, Zhou H, Li F, et al. Astrocyte transplantation for spinal cord injury: current status and perspective[J]. Brain Res Bull, 2014, 107: 18–30. doi: 10.1016/j.brainresbull.2014.05.003
|
| [21] |
Falnikar A, Li K, Lepore AC. Therapeutically targeting astrocytes with stem and progenitor cell transplantation following traumatic spinal cord injury[J]. Brain Res, 2015, 1619: 91–103. doi: 10.1016/j.brainres.2014.09.037
|
| [22] |
Nicaise C, Mitrecic D, Falnikar A, et al. Transplantation of stem cell-derived astrocytes for the treatment of amyotrophic lateral sclerosis and spinal cord injury[J]. World J Stem Cells, 2015, 7(2): 380–398. doi: 10.4252/wjsc.v7.i2.380
|
| [23] |
Chen C, Chan A, Wen H, et al. Stem and progenitor cell-derived astroglia therapies for neurological diseases[J]. Trends Mol Med, 2015, 21(11): 715–729. doi: 10.1016/j.molmed.2015.09.003
|
| [24] |
Martins-Macedo J, Lepore AC, Domingues HS, et al. Glial restricted precursor cells in central nervous system disorders: current applications and future perspectives[J]. Glia, 2021, 69(3): 513–531. doi: 10.1002/glia.23922
|
| [25] |
Han SSW, Liu Y, Tyler-Polsz C, et al. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter[J]. Glia, 2004, 45(1): 1–16. doi: 10.1002/glia.10282
|
| [26] |
Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord[J]. Exp Neurol, 2005, 194(1): 230–242. doi: 10.1016/j.expneurol.2005.02.020
|
| [27] |
Haas C, Neuhuber B, Yamagami T, et al. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration[J]. Exp Neurol, 2012, 233(2): 717–732. doi: 10.1016/j.expneurol.2011.11.002
|
| [28] |
Hill CE, Proschel C, Noble M, et al. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration[J]. Exp Neurol, 2004, 190(2): 289–310. doi: 10.1016/j.expneurol.2004.05.043
|
| [29] |
Wang JJ, Chuah MI, Yew DTW, et al. Effects of astrocyte implantation into the hemisected adult rat spinal cord[J]. Neuroscience, 1995, 65(4): 973–981. doi: 10.1016/0306-4522(94)00519-B
|
| [30] |
Pencalet P, Serguera C, Corti O, et al. Integration of genetically modified adult astrocytes into the lesioned rat spinal cord[J]. J Neurosci Res, 2006, 83(1): 61–67. doi: 10.1002/jnr.20697
|
| [31] |
Kliot M, Smith GM, Siegal JD, et al. Astrocyte-polymer implants promote regeneration of dorsal root fibers into the adult mammalian spinal cord[J]. Exp Neurol, 1990, 109(1): 57–69. doi: 10.1016/S0014-4886(05)80008-1
|
| [32] |
Kadoya K, Lu P, Nguyen K, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration[J]. Nat Med, 2016, 22(5): 479–487. doi: 10.1038/nm.4066
|
| [33] |
Olby NJ, Blakemore WF. Reconstruction of the glial environment of a photochemically induced lesion in the rat spinal cord by transplantation of mixed glial cells[J]. J Neurocytol, 1996, 25(1): 481–498. doi: 10.1007/BF02284817
|
| [34] |
Schackel T, Kumar P, Günther M, et al. Peptides and astroglia improve the regenerative capacity of alginate gels in the injured spinal cord[J]. Tissue Eng Part A, 2019, 25(7–8): 522–537. doi: 10.1089/ten.tea.2018.0082
|
| [35] |
Anderson KD. Targeting recovery: priorities of the spinal cord-injured population[J]. J Neurotrauma, 2004, 21(10): 1371–1383. doi: 10.1089/neu.2004.21.1371
|
| [36] |
Simpson LA, Eng JJ, Hsieh JTC, et al. The health and life priorities of individuals with spinal cord injury: a systematic review[J]. J Neurotrauma, 2012, 29(8): 1548–1555. doi: 10.1089/neu.2011.2226
|
| [37] |
Bernstein JJ, Goldberg WJ. Grafted fetal astrocyte migration can prevent host neuronal atrophy: comparison of astrocytes from cultures and whole piece donors[J]. Restor Neurol Neurosci, 1991, 2(4-6): 261–270. doi: 10.3233/RNN-1991-245615
|
| [38] |
Davies JE, Huang C, Proschel C, et al. Astrocytes derived from glial-restricted precursors promote spinal cord repair[J]. J Biol, 2006, 5(3): 7. doi: 10.1186/jbiol35
|
| [39] |
Davies JE, Pröschel C, Zhang N, et al. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury[J]. J Biol, 2008, 7(7): 24. doi: 10.1186/jbiol85
|
| [40] |
Fan C, Zheng Y, Cheng X, et al. Transplantation of D15A-expressing glial-restricted-precursor-derived astrocytes improves anatomical and locomotor recovery after spinal cord injury[J]. Int J Biol Sci, 2013, 9(1): 78–93. doi: 10.7150/ijbs.5626
|
| [41] |
Wu L, Li J, Chen L, et al. Combined transplantation of GDAsBMP and hr-decorin in spinal cord contusion repair[J]. Neural Regen Res, 2013, 8(24): 2236–2248. doi: 10.3969/j.issn.1673-5374.2013.24.003
|
| [42] |
Mitsui T, Shumsky JS, Lepore AC, et al. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry[J]. J Neurosci, 2005, 25(42): 9624–9636. doi: 10.1523/JNEUROSCI.2175-05.2005
|
| [43] |
Joosten EAJ, Veldhuis WB, Hamers FPT. Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury[J]. J Neurosci Res, 2004, 77(1): 127–142. doi: 10.1002/jnr.20088
|
| [44] |
Xu J, Bernreuther C, Cui Y, et al. Transplanted L1 expressing radial glia and astrocytes enhance recovery after spinal cord injury[J]. J Neurotrauma, 2011, 28(9): 1921–1937. doi: 10.1089/neu.2011.1783
|
| [45] |
Hofstetter CP, Holmström NAV, Lilja JA, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome[J]. Nat Neurosci, 2005, 8(3): 346–353. doi: 10.1038/nn1405
|
| [46] |
Macias MY, Syring MB, Pizzi MA, et al. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury[J]. Exp Neurol, 2006, 201(2): 335–348. doi: 10.1016/j.expneurol.2006.04.035
|
| [47] |
Hayashi K, Hashimoto M, Koda M, et al. Increase of sensitivity to mechanical stimulus after transplantation of murine induced pluripotent stem cell-derived astrocytes in a rat spinal cord injury model[J]. J Neurosurg Spine, 2011, 15(6): 582–593. doi: 10.3171/2011.7.SPINE10775
|
| [48] |
Goulão M, Ghosh B, Urban MW, et al. Astrocyte progenitor transplantation promotes regeneration of bulbospinal respiratory axons, recovery of diaphragm function, and a reduced macrophage response following cervical spinal cord injury[J]. Glia, 2019, 67(3): 452–466. doi: 10.1002/glia.23555
|
| [49] |
Li K, Javed E, Hala TJ, et al. Transplantation of glial progenitors that overexpress glutamate transporter GLT1 preserves diaphragm function following cervical SCI[J]. Mol Ther, 2015, 23(3): 533–548. doi: 10.1038/mt.2014.236
|
| [50] |
Jin Y, Shumsky JS, Fischer I. Axonal regeneration of different tracts following transplants of human glial restricted progenitors into the injured spinal cord in rats[J]. Brain Res, 2018, 1686: 101–112. doi: 10.1016/j.brainres.2018.01.030
|
| [51] |
Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord[J]. J Neurotrauma, 2013, 30(12): 1035–1052. doi: 10.1089/neu.2013.2915
|
| [52] |
Jin Y, Neuhuber B, Singh A, et al. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury[J]. J Neurotrauma, 2011, 28(4): 579–594. doi: 10.1089/neu.2010.1626
|
| [53] |
Davies SJA, Shih CH, Noble M, et al. Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury[J]. PLoS One, 2011, 6(3): e17328. doi: 10.1371/journal.pone.0017328
|
| [54] |
Shamblott MJ, Axelman J, Wang S, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells[J]. Proc Natl Acad Sci U S A, 1998, 95(23): 13726–13731. doi: 10.1073/pnas.95.23.13726
|
| [55] |
Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts[J]. Science, 1998, 282(5391): 1145–1147. doi: 10.1126/science.282.5391.1145
|
| [56] |
Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro[J]. Nat Biotechnol, 2000, 18(4): 399–404. doi: 10.1038/74447
|
| [57] |
Reubinoff BE, Itsykson P, Turetsky T, et al. Neural progenitors from human embryonic stem cells[J]. Nat Biotechnol, 2001, 19(12): 1134–1140. doi: 10.1038/nbt1201-1134
|
| [58] |
Zhang S, Wernig M, Duncan ID, et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells[J]. Nat Biotechnol, 2001, 19(12): 1129–1133. doi: 10.1038/nbt1201-1129
|
| [59] |
Chen H, Qian K, Chen W, et al. Human-derived neural progenitors functionally replace astrocytes in adult mice[J]. J Clin Invest, 2015, 125(3): 1033–1042. doi: 10.1172/JCI69097
|
| [60] |
Lu P, Ceto S, Wang Y, et al. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS[J]. J Clin Invest, 2017, 127(9): 3287–3299. doi: 10.1172/JCI92955
|
| [61] |
Lien BV, Tuszynski MH, Lu P. Astrocytes migrate from human neural stem cell grafts and functionally integrate into the injured rat spinal cord[J]. Exp Neurol, 2019, 314: 46–57. doi: 10.1016/j.expneurol.2019.01.006
|
| [62] |
Roybon L, Lamas NJ, Garcia-Diaz A, et al. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes[J]. Cell Reports, 2013, 4(5): 1035–1048. doi: 10.1016/j.celrep.2013.06.021
|
| [63] |
Haidet-Phillips AM, Roybon L, Gross SK, et al. Gene profiling of human induced pluripotent stem cell-derived astrocyte progenitors following spinal cord engraftment[J]. Stem Cells Transl Med, 2014, 3(5): 575–585. doi: 10.5966/sctm.2013-0153
|
| [64] |
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors[J]. Cell, 2006, 126(4): 663–676. doi: 10.1016/j.cell.2006.07.024
|
| [65] |
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors[J]. Cell, 2007, 131(5): 861–872. doi: 10.1016/j.cell.2007.11.019
|
| [66] |
Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells[J]. Science, 2007, 318(5858): 1917–1920. doi: 10.1126/science.1151526
|
| [67] |
Li K, Javed E, Scura D, et al. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury[J]. Exp Neurol, 2015, 271: 479–492. doi: 10.1016/j.expneurol.2015.07.020
|
| [68] |
Qian K, Huang H, Peterson A, et al. Sporadic ALS astrocytes induce neuronal degeneration in vivo[J]. Stem Cell Reports, 2017, 8(4): 843–855. doi: 10.1016/j.stemcr.2017.03.003
|
| [69] |
Krencik R, Weick JP, Liu Y, et al. Specification of transplantable astroglial subtypes from human pluripotent stem cells[J]. Nat Biotechnol, 2011, 29(6): 528–534. doi: 10.1038/nbt.1877
|
| [70] |
Krencik R, Zhang S. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells[J]. Nat Protoc, 2011, 6(11): 1710–1717. doi: 10.1038/nprot.2011.405
|
| [71] |
Li X, Tao Y, Bradley R, et al. Fast generation of functional subtype astrocytes from human pluripotent stem cells[J]. Stem Cell Reports, 2018, 11(4): 998–1008. doi: 10.1016/j.stemcr.2018.08.019
|
| [72] |
Tchieu J, Calder EL, Guttikonda SR, et al. NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells[J]. Nat Biotechnol, 2019, 37(3): 267–275. doi: 10.1038/s41587-019-0035-0
|
| [73] |
Bradley RA, Shireman J, McFalls C, et al. Regionally specified human pluripotent stem cell-derived astrocytes exhibit different molecular signatures and functional properties[J]. Development, 2019, 146(13): dev170910. doi: 10.1242/dev.170910
|
| [74] |
Kumamaru H, Kadoya K, Adler AF, et al. Generation and post-injury integration of human spinal cord neural stem cells[J]. Nat Methods, 2018, 15(9): 723–731. doi: 10.1038/s41592-018-0074-3
|
| [75] |
van Middendorp JJ, Allison H, Cowan K, et al. Top ten research priorities for spinal cord injury[J]. Lancet Neurol, 2014, 13(12): 1167. doi: 10.1016/S1474-4422(14)70253-4
|
| [76] |
Courtine G, Bunge MB, Fawcett JW, et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans?[J]. Nat Med, 2007, 13(5): 561–566. doi: 10.1038/nm1595
|
| [77] |
Kwon BK, Streijger F, Hill CE, et al. Large animal and primate models of spinal cord injury for the testing of novel therapies[J]. Exp Neurol, 2015, 269: 154–168. doi: 10.1016/j.expneurol.2015.04.008
|
| [78] |
Kwon BK, Soril LJJ, Bacon M, et al. Demonstrating efficacy in preclinical studies of cellular therapies for spinal cord injury - How much is enough?[J]. Exp Neurol, 2013, 248: 30–44. doi: 10.1016/j.expneurol.2013.05.012
|