| Citation: | Jiang Nancy, Larrazabal Ramiro, Alsunbul Waleed, Lu Jian-Qiang. Angiosarcomatous component in gliosarcoma: case report and consideration of diagnostic challenge and hemorrhagic propensity[J]. The Journal of Biomedical Research, 2020, 34(2): 143-148. DOI: 10.7555/JBR.33.20190080 |
Gliosarcoma is a glioblastoma variant that consists of alternating gliomatous and sarcomatous components. The sarcomatous component is commonly fibroblastic, and rarely angiosarcomatous[1–2]. Despite the rarity, gliosarcoma with angiosarcomatous component may mimic some other brain malignant tumors and vascular lesions, which makes the preoperative diagnosis challenging[2–4]. Moreover, the rich vascularity and adhesive properties of the angiosarcomatous lesion could result in an increased risk of intraoperative hemorrhage. Here we report a unique case of gliosarcoma with an angiosarcomatous component in which surgical resections required greater efforts to control intraoperative bleeding and to maintain hemostasis.
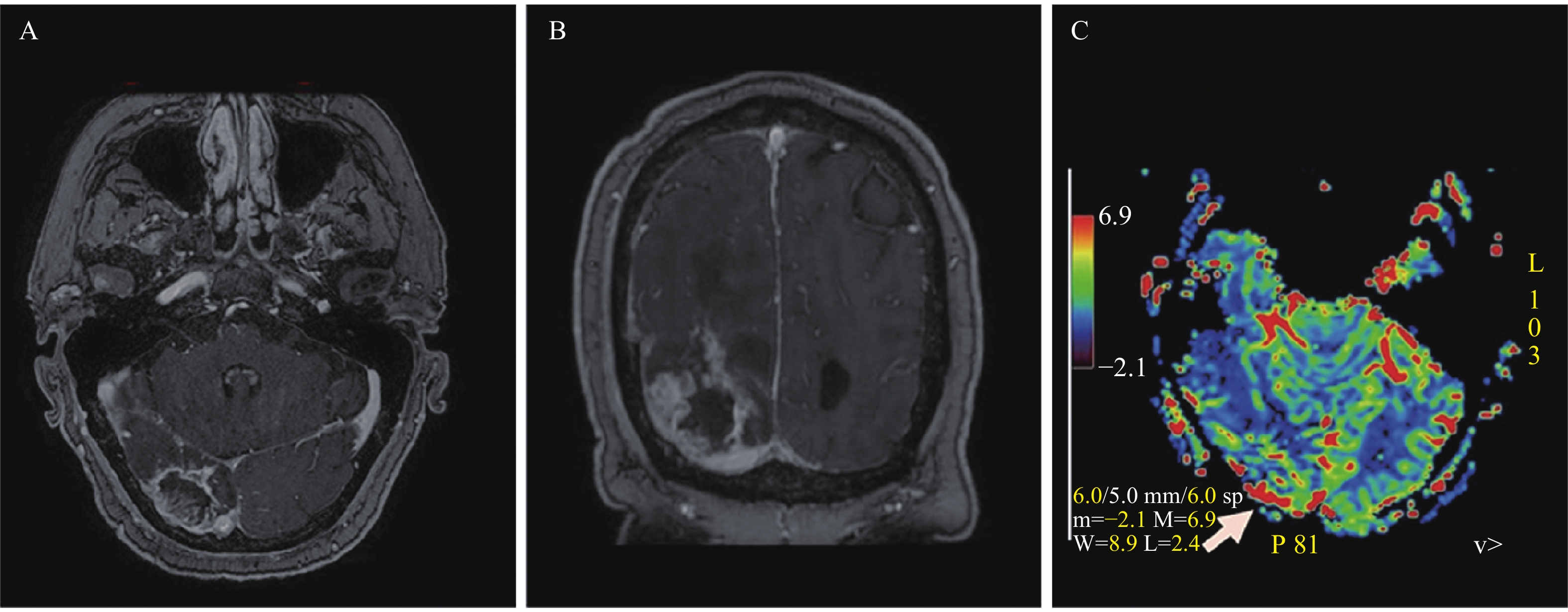
The patient was a 61-year-old male who presented with 4 weeks of peripheral vision loss and difficulty walking. His past medical history was not contributory. Physical examination revealed left homonymous hemianopsia and hesitant gait likely due to his vision deficit. The brain magnetic resonance imaging (MRI) showed a peripherally-located, well-demarcated and thick-walled lesion in the right occipital lobe with multiple flow voids noted. On post-contrast T1-weighted images, the lesion demonstrated not only rim-like enhancement but also intratumoral strip (paliform) and nodular enhancement. On diffusion-weighted images, the paliform and nodular components exhibited areas with restricted diffusion. The lesion appeared to be inseparable from the right tentorial leaflet (Fig. 1). There was significant mass effect including a 3 mm leftward midline shift, partial effacement of right occipital horn and moderate perilesional edema. Systemic workup for metastasis in this patient was negative.
The patient subsequently underwent a right occipital craniotomy for resection of the mass. Intraoperatively, the mass was grayish and reddish alternately in color with a hard capsule and focally rich vascularity; composed of soft and hard components. The capsule was adhesive to the surrounding brain tissue and inseparable from the right tentorial leaflet. There was considerable blood oozing from the mass, which required extensive irrigation and using multiple Surgicels and FloSeals. Consequently, the patient experienced a 1.5 L blood loss during the resection and the neurosurgical team elected to stop after partial resection of the mass.
Pathological examination of the resected tissue revealed a gliosarcoma, WHO grade Ⅳ, with alternating gliomatous and sarcomatous components (Fig. 2). The sarcomatous component was composed of abundant blood vessels with focal microhemorrhages. Immunohistochemically, this tumor was negative for IDH1(R132H) immunostaining; the sarcomatous component was largely positive for the endothelial markers including CD34, CD31, and Factor Ⅷ, which suggested angiosarcomatous features. Analysis of the tumor DNA by polymerase chain reaction showed that O6-methylguanine DNA methyltransferase promoter was unmethylated.
The postoperative MRI exhibited the residual tumor and surgical cavity with focal hemorrhage (despite greater efforts to control bleeding during the operation) and fluid collection (Fig. 3). Postoperatively, the patient received radiation treatment using an isocentric field technique, with 6 MV photons, and a total dose of 60 Gy in 30 fractions (2 Gy per fraction) to the surgical bed, based on clinical target volume identified on gadolinium-enhanced T1-weighted MRI, with 2 cm margin. He was concurrently treated with temozolomide for 6 weeks followed by adjuvant temozolomide. Despite his persistent hemianopsia, he was clinically stable without significant tumor growth on the follow-up MRIs (not shown) and progression-free for 8 months. At 9 months after the first resection, the patient presented with a syncopal episode. MRI showed a mild increase in the size and intensity of the enhancing tumor (Fig. 4A).
The patient underwent a second resection of the recurrent/residual tumor. Intraoperatively, the tumor was again bloody with focally rich vascularity, and its resection required multiple rounds of peroxide soaked cottonoids and bipolar electrosurgery to obtain hemostasis. Pathological examination of the second resection exhibited essentially similar features of a gliosarcoma with an angiosarcomatous component and intermingled treatment-associated changes (Fig. 4B). A postoperative head computed tomography showed postoperative changes with focal hemorrhage (Fig. 4C).
Gliosarcomas account for approximately 2% of all glioblastomas[1]. The cellular origin of the sarcomatous component in gliosarcomas remains unclear. Originally gliosarcoma was thought to be a collision tumor with a separate astrocytic component and independent development of the sarcomatous portion from the proliferating vessels, but some studies have supported another hypothesis that the sarcomatous differentiation results from a phenotypic change in the glioblastoma cells rather than an indication of the coincidental development of two separate neoplasms[1,5]. Despite the inconclusive pathogenesis of sarcomatous component, the proliferating vessels are commonly seen in gliosarcomas and become predominant in the angiosarcomatous component of gliosarcomas.
There is another rare entity of primary central nervous system (CNS) angiosarcoma that should be diagnosed in the absence of glial component, in addition to secondary CNS angiosarcomas[4]. The primary CNS angiosarcoma are originating from endothelial cells of the blood vessels, and arising from the cerebral parenchyma in most reported cases or meninges in a few other cases[4]. The clinicopathological features and properties of primary cerebral angiosarcomas are similar to those of the angiosarcomatous component in gliosarcomas. As the majority of reported CNS angiosarcomas present with hemorrhage[4], it is expected that an angiosarcomatous component in gliosarcomas is associated with hemorrhagic propensity. In our present case, MR images of the tumor demonstrated avid enhancement, restricted diffusion, prominent flow voids, and significant peritumoral edema, which are all features of high-grade glioma typically with rich vascularity. In addition, the preoperative MR perfusion not only helped to identify the tumor progression but also demonstrated increased CBV of the tumor, which is also suggestive of increased vascularity. However, the MR perfusion study may have underestimated the degree of vascularity of the tumor, compared to the intraoperative and pathological findings.
Angiosarcomatous features have been reported exceptionally in other primary brain tumors including a case of oligodendroglioma with mixed fibrosarcomatous and angiosarcomatous features[6] and another case of recurrent subependymoma with angiosarcoma-like overgrowth[5]. So far, there has been only one reported case of gliosarcoma with frankly angiosarcomatous features[2]. That tumor was described as a T1-isointense and T2-hyperintense lesion with a large cystic space and heterogeneous contrast enhancement; it was severely bleeding during the tumor resection[2]. In contrast, the tumor in our present case demonstrated characteristic features such as asymmetrically thick wall, prominent flow voids, rim-like enhancement as well as multiple enhancing intratumoral strips and nodular components. The characteristic enhancing intratumoral strip and nodular components have been described in less than half of gliosarcomas without frank angiosarcomatous features and likely correspond to abundant vascular proliferation within the tumor[7]. In our present case, the tumor hemorrhage was seen during and after the first and second resections.
Unlike infiltrative glioblastomas, gliosarcomas are usually well-circumscribed, rim-enhancing lesions with intratumoral paliform and nodular components on neuroimaging. The differential diagnosis for a gliosarcoma with angiosarcomatous component includes other glioblastoma variants with hypervascular features, metastatic tumors, brain abscess, and some vascular lesions[2–4,8–9]. Gliosarcomas with an angiosarcomatous components may be radiologically distinguishable from these entities by characteristic features including peripheral location, well-demarcated margin, as well as characteristic intratumoral strips and nodular enhancing components. Nevertheless, the pathological examination of a reasonable tissue sample is the key to diagnose the angiosarcomatous component in gliosarcomas.
Gliosarcomas are usually treated with surgical resections followed by radiotherapy with or without chemotherapy, so are primary CNS angiosarcomas. With the treatment of surgical resection followed by radiotherapy and chemotherapy, the generally poor prognosis despite considerable variation is noted in both primary CNS angiosarcomas (with the median survival of 8 months[4]) and gliosarcomas (with the median overall survival of 13.9 and 7.9 months, respectively, in two recently published studies[10–11]; the median progression-free survival 7.9 months in the former study[10]). The previously reported patient with an angiosarcomatous component in a gliosarcoma was free from recurrence during a 7-month follow-up after the same treatment. Our present patient was free from recurrence for 8 months but had the tumor recurrence at 9 months when the second resection was required. Therefore, it seems that gliosarcomas with an angiosarcomatous component are prognostically of no significant difference from other gliosarcomas although the angiosarcomatous component is commonly associated with intraoperative and/or postoperative hemorrhage. A recent study by Smith et al showed that in the rat in vivo model of 9L orthotopic gliosarcomas, overall survival was significantly prolonged with neurosurgical delivery of etoposide and temozolomide into the surgical resection cavity[12]. This approach may play an important role in the future treatment of gliosarcomas including those with angiosarcomatous features.
In conclusion, awareness of perplexing radiographically heterogeneity of gliosarcoma and the increased hemorrhage risk of an angiosarcomatous component in gliosarcoma is important for the patient's preoperative diagnosis and surgical management.
We thank Dr. Sunjay V. Sharma, the multidisciplinary neuro-oncology team, and other medical professionals at Hamilton Health Sciences for providing clinical care to the patient.
| [1] |
Kleihues P, Burger PC, Aldape KD, et al. Glioblastoma[M]//Louis DN, Ohgaki H, Wiestler OD, et al. World Health Organization Classification of Tumours of the Central Nervous System. 4th ed. Lyon: IARC, 2007: 48–49.
|
| [2] |
Shintaku M, Miyaji K, Adachi Y. Gliosarcoma with angiosarcomatous features: a case report[J]. Brain Tumor Pathol, 1998, 15(2): 101–105. doi: 10.1007/BF02478891
|
| [3] |
Matsuno A, Nagashima T, Tajima Y, et al. A diagnostic pitfall: angiosarcoma of the brain mimicking cavernous angioma[J]. J Clin Neurosci, 2005, 12(6): 688–691. doi: 10.1016/j.jocn.2004.08.028
|
| [4] |
La Corte E, Acerbi F, Schiariti M, et al. Primary central nervous system angiosarcoma: a case report and literature review[J]. Neuropathology, 2015, 35(2): 184–191. doi: 10.1111/neup.12178
|
| [5] |
Louis DN, Hedley-Whyte ET, Martuza RL. Sarcomatous proliferation of the vasculature in a subependymoma[J]. Acta Neuropathol, 1989, 78(3): 332–335. doi: 10.1007/BF00687765
|
| [6] |
Pasquier B, Couderc P, Pasquier D, et al. Sarcoma arising in oligodendroglioma of the brain. A case with intramedullary and subarachnoid spinal metastases[J]. Cancer, 1978, 42(6): 2753–2758. doi: 10.1002/1097-0142(197812)42:6<2753::AID-CNCR2820420634>3.0.CO;2-K
|
| [7] |
Han L, Zhang X, Qiu S, et al. Magnetic resonance imaging of primary cerebral gliosarcoma: a report of 15 cases[J]. Acta Radiol, 2008, 49(9): 1058–1067. doi: 10.1080/02841850802314796
|
| [8] |
Khanna A, Venteicher AS, Walcott BP, et al. Glioblastoma mimicking an arteriovenous malformation[J]. Front Neurol, 2013, 4: 144.
|
| [9] |
Guo AC, Provenzale JM, Cruz LCH Jr, et al. Cerebral abscesses: investigation using apparent diffusion coefficient maps[J]. Neuroradiology, 2001, 43(5): 370–374. doi: 10.1007/s002340000519
|
| [10] |
Adeberg S, Bernhardt D, Harrabi SB, et al. Radiotherapy plus concomitant temozolomide in primary gliosarcoma[J]. J Neurooncol, 2016, 128(2): 341–348. doi: 10.1007/s11060-016-2117-x
|
| [11] |
Jain A, Correia J, Schweder P, et al. Analysis of outcomes of multidisciplinary management of gliosarcoma: a single-center study, 2000-2013[J]. World Neurosurg, 2017, 106: 30–36. doi: 10.1016/j.wneu.2017.06.073
|
| [12] |
Smith SJ, Tyler BM, Gould T, et al. Overall survival in malignant glioma is significantly prolonged by neurosurgical delivery of etoposide and temozolomide from a thermo-responsive biodegradable paste[J]. Clin Cancer Res, 2019, 25(16): 5094–5106. doi: 10.1158/1078-0432.CCR-18-3850
|
| 1. | Lee C, Kim MS, Hwang JH, et al. Venous ectasia preceding intra-tumoral hemorrhage in a case of gliosarcoma with transverse sinus involvement. J Surg Case Rep, 2023, 2023(7): rjad429. DOI:10.1093/jscr/rjad429 |
| 2. | Jiang NN, Larrazabal R, Alsunbul W, et al. Multiple pathological components in gliosarcoma. J Biomed Res, 2020, 35(5): 408-410. DOI:10.7555/JBR.34.20200089 |